Enantioselective Synthesis of Tetrahydro-1H-1,3-methanocarbazoles by Formal (3 + 3)-Cycloaddition Using Bicyclo[1.1.0]butanes
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Asymmetric synthesis presents many challenges, with the selective formation of chiral bridged polyheterocycles being a notable example. Cycloadditions using bicyclo[1.1.0]butanes (BCB) offer a promising solution along those lines, yet, despite significant advances in that emerging area, asymmetric control has remained limited thus far. Here, we describe an organocatalytic, enantioselective formal (3 + 3)-cycloaddition of BCBs with 1H-indol-3-yl((hetero)aryl)methanol derivatives. This approach enables the rapid and efficient synthesis of chiral tetrahydro-1H-1,3-methanocarbazole derivatives (34 examples) from readily available starting materials, with very good stereochemical control (up to 98:2 er). Successful scale-up experiments and product modification demonstrated the potential of this methodology. Control experiments and DFT calculations provide insights into the mechanistic pathway.
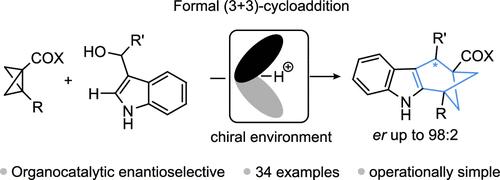
双环[1.1.0]丁烷正(3 + 3)-环加成法对映选择性合成四氢- 1h -1,3-甲烷咔唑
不对称合成提出了许多挑战,手性桥接多杂环的选择性形成就是一个显著的例子。使用双环[1.1.0]丁烷(BCB)的环加成提供了一个很有前途的解决方案,然而,尽管在这一新兴领域取得了重大进展,但迄今为止,不对称控制仍然有限。在这里,我们描述了一个有机催化的,对映选择性的bcb与1h -吲哚-3-基(杂)芳基)甲醇衍生物的形式(3 + 3)环加成。这种方法可以从现成的原料中快速有效地合成手性四氢- 1h -1,3-甲烷咔唑衍生物(34个例子),具有非常好的立体化学控制(高达98:2 er)。成功的放大实验和产品修改证明了这种方法的潜力。控制实验和DFT计算提供了对机制途径的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


