Sporothrix brasiliensis Gp70 is a cell wall protein required for adhesion, proper interaction with innate immune cells, and virulence
IF 6.2
Q1 Immunology and Microbiology
引用次数: 0
Abstract
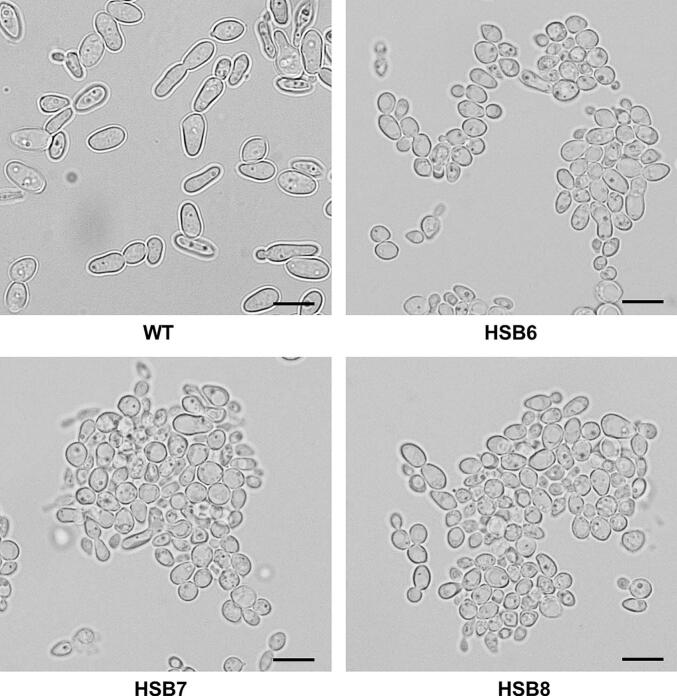
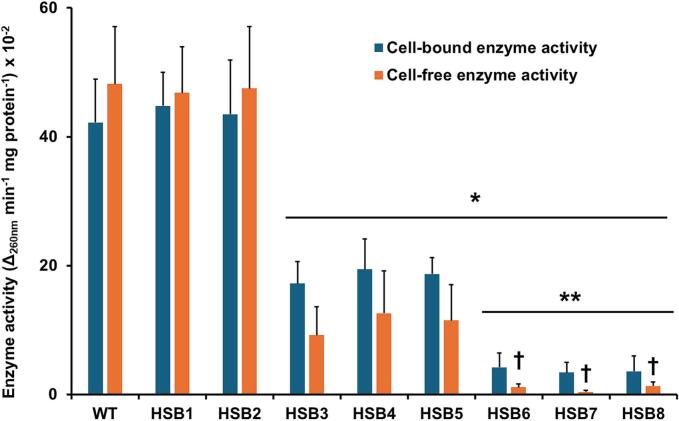
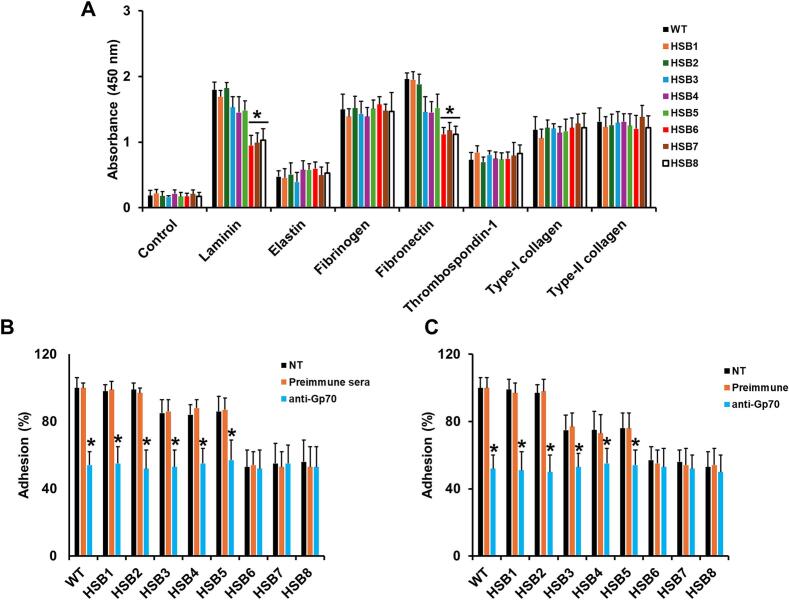
Sporothrix brasiliensis is one of the leading etiological agents of sporotrichosis, a cutaneous and subcutaneous mycosis worldwide distributed. This organism has been recently associated with epidemic outbreaks in Brazil. Despite the medical relevance of this species, little is known about its virulence factors, and most of the information on this subject is extrapolated from Sporothrix schenckii. Here, we generated S. brasiliensis mutants, where GP70 was silenced. In S. schenckii, this gene encodes a glycoprotein with adhesive properties required for virulence. The S. brasiliensis GP70 silencing led to an abnormal cellular phenotype, with smaller, round yeast-like cells that aggregate. Cell aggregation was disrupted with glucanase, suggesting this phenotype is linked to changes in the cell wall. The cell wall characterization confirmed changes in the structural polysaccharide β-1,3-glucan, which increased in quantity and exposure at the cell surface. This was accompanied by a reduction in protein content and N-linked glycans. Mutant strains with high GP70-silencing levels showed minimal levels of 3-carboxy-cis,cis-muconate cyclase activity, this glycoprotein's predicted enzyme function, and decreased ability to bind laminin and fibronectin. These phenotypical changes coincided with abnormal interaction with human peripheral blood mononuclear cells, where production of IL-1β, IL-17, and IL-22 was reduced and the strong dependence on cytokine stimulation via mannose receptor was lost. Phagocytosis by monocyte-derived macrophages was increased and virulence attenuated in a Galleria mellonella larvae. In conclusion, Gp70 is an abundant cell wall glycoprotein in S. brasiliensis that contributes to virulence and proper interaction with innate immnune cells.
巴西孢子菌Gp70是一种细胞壁蛋白,是粘附、与先天免疫细胞相互作用和毒力所必需的。
巴西孢子丝虫是孢子毛病的主要病原体之一,孢子毛病是一种分布在世界各地的皮肤和皮下真菌病。这种生物最近与巴西的流行病爆发有关。尽管该物种具有医学意义,但对其毒力因素知之甚少,有关该主题的大多数信息都是从申克孢子丝菌推断出来的。在这里,我们产生了GP70被沉默的巴西葡萄球菌突变体。在申氏球菌中,该基因编码一种糖蛋白,该糖蛋白具有毒性所需的粘附特性。巴西芽孢杆菌GP70沉默导致异常的细胞表型,具有较小的圆形酵母样细胞聚集。葡聚糖酶破坏了细胞聚集,表明这种表型与细胞壁的变化有关。细胞壁表征证实了结构多糖β-1,3-葡聚糖的变化,其在细胞表面的数量和暴露量增加。这伴随着蛋白质含量和n链聚糖的减少。具有高gp70沉默水平的突变菌株显示3-羧基顺式、顺式黏液酸环化酶活性(该糖蛋白的预测酶功能)水平极低,并且结合层粘连蛋白和纤维连接蛋白的能力下降。这些表型变化与人外周血单个核细胞的异常相互作用相吻合,其中IL-1β, IL-17和IL-22的产生减少,并且失去了对通过甘露糖受体刺激的细胞因子的强烈依赖。单核细胞源性巨噬细胞的吞噬作用增强,毒力减弱。综上所述,Gp70是巴西螺中丰富的细胞壁糖蛋白,参与了巴西螺的毒力和与先天免疫细胞的适当相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Surface
Immunology and Microbiology-Applied Microbiology and Biotechnology
CiteScore
6.10
自引率
0.00%
发文量
18
审稿时长
49 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


