Afatinib and Necitumumab in EGFR-Mutant NSCLC with Acquired Resistance to Tyrosine Kinase Inhibitors
IF 3.5
Q2 ONCOLOGY
引用次数: 0
Abstract
Introduction
Although tyrosine kinase inhibitors (TKIs) are effective against NSCLC harboring sensitizing EGFR gene mutations, acquired resistance is inevitable. Preclinical studies suggest that combining EGFR TKI and monoclonal antibody therapies may have activity in EGFR-mutated NSCLC that has progressed on TKI therapy alone. Therefore, we prospectively evaluated afatinib plus necitumumab in patients with EGFR-mutated NSCLC.
Methods
This was a phase 1, dose-escalation, dose-expansion trial assessing the safety and efficacy of afatinib plus necitumumab. Patients had advanced or metastatic EGFR-mutated NSCLC with progression after (1) first-generation TKI if T790M negative, (2) subsequent line third-generation TKI if T790M positive, or (3) third-generation TKI in the first-line setting. Dose-escalation followed a 3+3 design. The primary end point of dose-expansion was objective response rate.
Results
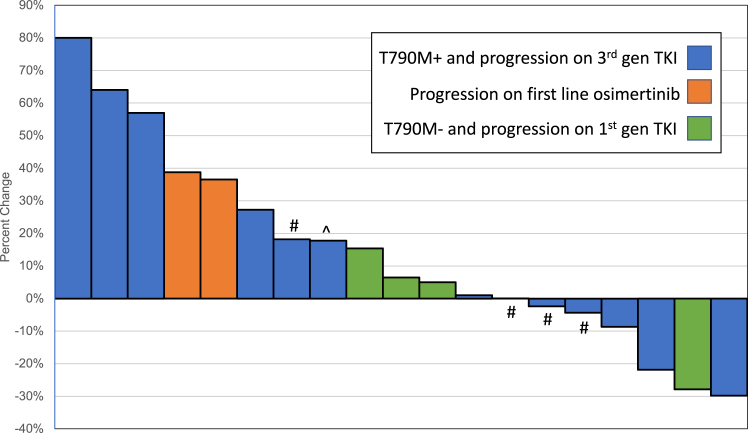
A total of 22 patients with EGFR-mutated NSCLC were enrolled. The maximum tolerated dose was afatinib 40 mg oral daily plus necitumumab 600 mg intravenous on days 1 and 15 every 28 days. There were no grade 4 to 5 adverse events observed, and seven patients (32%) experienced grade 3 treatment-related adverse events (three rash; one each oral mucositis, diarrhea, headache, ventricular arrhythmia, and tachycardia). In the entire cohort, there were no responses observed, the median progression-free survival was 1.8 months, and the disease control rate was 36% but varied between the subgroups.
Conclusions
Afatinib plus necitumumab was safe but had limited activity in patients with EGFR-mutated NSCLC. Biomarker studies may identify patient subgroups that are more likely to benefit.
阿法替尼和奈西单抗治疗对酪氨酸激酶抑制剂获得性耐药的egfr突变型NSCLC。
虽然酪氨酸激酶抑制剂(TKIs)对含有致敏性EGFR基因突变的NSCLC有效,但获得性耐药是不可避免的。临床前研究表明,联合EGFR TKI和单克隆抗体治疗可能对单独TKI治疗进展的EGFR突变的NSCLC有活性。因此,我们前瞻性地评估了阿法替尼联合奈西单抗在egfr突变的NSCLC患者中的应用。方法:这是一项1期、剂量递增、剂量扩展试验,评估阿法替尼联合纳西单抗的安全性和有效性。晚期或转移性egfr突变的NSCLC患者在(1)T790M阴性时接受第一代TKI治疗,(2)T790M阳性时接受第三代TKI治疗,或(3)一线患者接受第三代TKI治疗后进展。剂量递增遵循3+3设计。剂量扩大的主要终点为客观有效率。结果:共有22例egfr突变的NSCLC患者入组。最大耐受剂量为每日口服阿法替尼40毫克,第1天和第15天静脉注射纳西单抗600毫克,每28天一次。没有观察到4至5级不良事件,7名患者(32%)经历了3级治疗相关不良事件(3例皮疹;口腔黏膜炎、腹泻、头痛、室性心律失常、心动过速各1例。在整个队列中,未观察到任何反应,中位无进展生存期为1.8个月,疾病控制率为36%,但在亚组之间存在差异。结论:Afatinib + necitumumab对egfr突变的NSCLC患者是安全的,但活性有限。生物标志物研究可以确定更有可能受益的患者亚组。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


