Detecting Reactive Products in Carbon Capture Polymers with Chemical Shift Anisotropy and Machine Learning
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
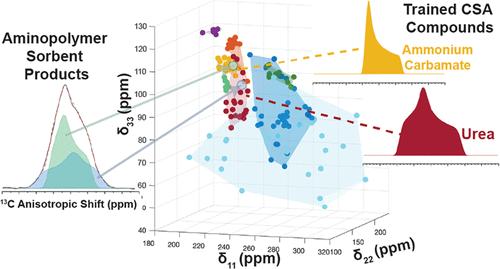
Aminopolymers are attractive sorbents for CO2 direct air capture applications due to their high density of amine groups, which can readily react with atmospheric levels of CO2 to form chemisorbed species. The identity of these chemisorbed species and the functional groups that form upon oxidative degradation depends on both material properties and processing conditions, forming a variety of carbonyl-type sites such as ammonium carbamates, bicarbonates, carbonates, carbamic acids, ureas, and amides. 13C solid-state nuclear magnetic resonance (NMR) is often used to help elucidate the identity of these reacted species, but it is challenging due to the narrow chemical shift range of carbonyl sites. Herein, we demonstrate the application of a two-dimensional (2D) chemical shift anisotropy (CSA) recoupling pulse sequence (ROCSA) to obtain CSA tensor values at each isotropic chemical shift, overcoming limitations of isotropic peak resolution. CSA tensor values describe the local chemical environment and can readily differentiate between the chemisorbed and degradation products. To aid identification, we also developed a k-nearest neighbor (kNN) classification model to distinguish the functional groups via their CSA tensor parameters. This methodology was demonstrated on poly(ethylenimine) in γ-Al2O3 exposed to CO2 and showed that the chemisorbed products are ammonium carbamate and a mixed carbamate–carbamic acid species. The sample was analyzed again after desorption at 100 °C inducing mild degradation, and the remaining products were strongly bound carbamate and urea species. The combination of 2D CSA measurements coupled with a kNN classification model enhances the ability to accurately identify chemisorbed or degradation products in complex carbon capture materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


