Temperature Dependence of Photocatalytic Water Splitting under Visible Light Irradiation over Ir- and Sb-Codoped SrTiO3:Al
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Temperature dependence of photocatalytic water splitting over CrOx/Rh and CoOOH cocatalyst-coloaded SrTiO3:Al codoped with Ir and Sb single-particulate photocatalysts (CrOx/Rh/SrTiO3:Ir,Sb,Al/CoOOH) under visible light irradiation was investigated. When the reaction temperature was increased from 280 to 340 K, the activity became low, and degradation became significant. Moreover, the activity was partially revived with lowering reaction temperature from 340 to 290 K. These results suggest that reversible and irreversible negative factors worked with rising reaction temperature. The temperature dependence was caused by the working of the negative factors such as the irreversible degradation of photocatalyst · cocatalyst and the reversible increase of recombination probability of photogenerated carriers more than the positive factor such as increasing the rate of surface reaction. X-ray photoelectron spectra revealed that detachment and/or aggregation of CoOOH loaded on SrTiO3:Ir,Sb,Al brought the irreversible degradation of the photocatalyst material at 340 K. In contrast to it, the change of a CrOx shell in the CrOx/Rh cocatalyst was not observed, suggesting that the reverse reactions to form water from evolved H2 and O2 and to reduce O2 were not enhanced even though reaction temperature was risen. On the other hand, the photoluminescence intensity of Eu3+ as a guest doped to SrTiO3:Ir,Sb,Al as a host due to band gap excitation of SrTiO3 became low with rising reaction temperature, suggesting that the recombination probability as a nonradiative deactivation of photogenerated carriers in the host of SrTiO3 increased. Therefore, it was suggested that the negative factors such as degradation of a CoOOH cocatalyst and increasing recombination probability of photogenerated carriers caused the decrease of the photocatalytic water-splitting activity of CrOx/Rh/SrTiO3:Ir,Sb,Al/CoOOH.
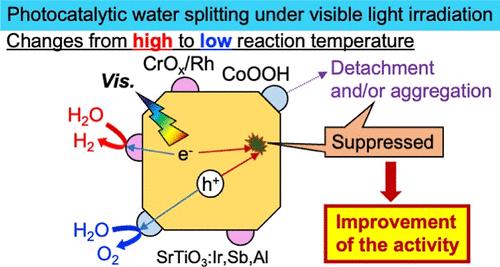
Ir和sb共掺杂SrTiO3:Al在可见光下光催化水分解的温度依赖性
研究了crx /Rh和CoOOH共催化剂负载SrTiO3:Al共掺杂Ir和Sb单颗粒光催化剂(crx /Rh/SrTiO3:Ir,Sb,Al/CoOOH)在可见光下光催化水分解的温度依赖性。当反应温度从280 ~ 340 K升高时,活性降低,降解明显。当反应温度从340 K降低到290 K时,活性有所恢复。这些结果表明,可逆和不可逆的负面因素随反应温度的升高而起作用。温度依赖性主要是由于光催化剂·助催化剂的不可逆降解和光生载体的复合概率的可逆增加等消极因素的作用大于表面反应速率的提高等积极因素的作用。x射线光电子能谱显示,负载在SrTiO3:Ir,Sb,Al上的CoOOH分离和/或聚集导致了光催化剂材料在340 K下的不可逆降解。与此相反,在CrOx/Rh共催化剂中没有观察到CrOx壳层的变化,说明即使反应温度升高,也没有增强由H2和O2生成水和还原O2的逆反应。另一方面,由于SrTiO3的带隙激发,Eu3+作为客体掺杂到SrTiO3:Ir,Sb,Al作为寄主的光致发光强度随着反应温度的升高而降低,表明SrTiO3寄主中光生载流子作为非辐射失活的重组概率增加。因此,认为CoOOH助催化剂的降解和光生载体的重组概率增加等负面因素导致了CrOx/Rh/SrTiO3:Ir,Sb,Al/CoOOH光催化分解水活性的降低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


