Propane Dehydrogenation on Pt Single-Atom and Pt4 and Pt3Sn Single-Cluster Supported on g-C3N4: A Theoretical Study
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
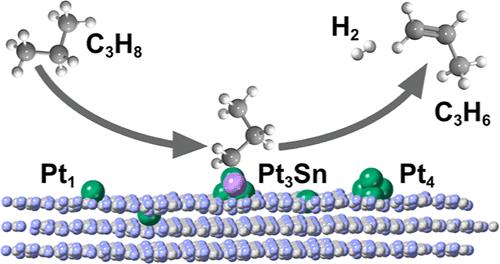
Propane dehydrogenation (PDH) is one of the most widely used processes to produce propylene. Platinum is an effective catalyst for PDH. However, metallic Pt facilitates deep dehydrogenation and coke formation. In contrast, small subnanoparticles and, at the limit, single-atom catalysts have shown high reactivity, prevent coke formation, and are much cheaper. Graphitic carbon nitride (g-C3N4) has been used as a support for several catalytic and electrocatalytic processes. In this work, three Pt-based catalysts, single-atom (Pt1), single-cluster (Pt4), and bimetallic Pt3Sn anchored on the heptazine units of g-C3N4 were modeled using the density functional theory approach in conjunction with microkinetic analysis. Ab initio molecular dynamics has also been used to ensure the stability of the systems. All three systems showed good activity for PDH. However, we have shown that the conversion is only satisfactory at high temperatures, according to the thermodynamic and kinetic requirements, due to the high cost of propene desorption. On the other hand, the energy barriers for the deep dehydrogenation side reaction remain similar as the temperature increases. Substituting a Pt atom by Sn in Pt4 to give a bimetallic subnanometric Pt3Sn cluster facilitates propene desorption and inhibits deep dehydrogenation and subsequent coke formation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


