Naphthalene Decomposition on Fe(110)─Adsorption, Dehydrogenation, Surface Carbon Formation and the Influence of Coadsorbed Oxygen
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
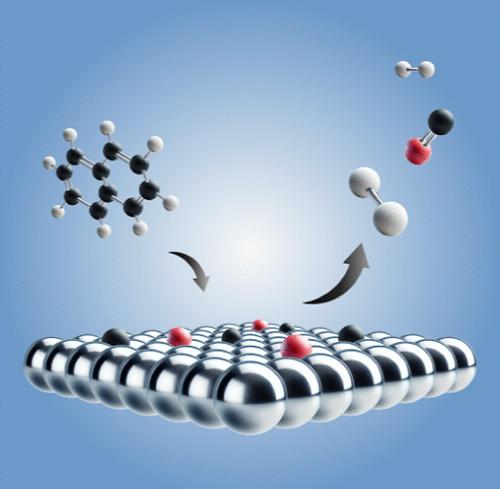
Tar is an undesirable byproduct of biomass gasification, which can be removed through catalytic reforming to syngas components. Iron is a promising, abundant alternative to highly active but toxic nickel catalysts. The results observed so far in catalytic studies with iron have been mixed. In this paper, the decomposition of naphthalene, a representative model compound of tar, was studied on the catalytic Fe(110) surface using temperature-programmed desorption (TPD), sum frequency generation spectroscopy (SFG), and X-ray photoelectron spectroscopy (XPS). Napthalene adsorption, dehydrogenation and the formation of surface carbon were investigated, as well as the influence of oxygen. In comparison with previous studies on Ni(111), a similar dehydrogenation activity was found for Fe(110) with two main H2 TPD peaks at 450 and 550 K. The reaction of naphthalene on Fe(110) resulted in the predominant formation of carbidic and atomically adsorbed carbon on the surface, which did not dissolve into the bulk even at high temperatures. A moderately carbon-covered surface was shown to still be active toward naphthalene decomposition. Similarly to Ni(111), large amounts of oxygen inhibited the reaction but, at low oxygen doses, very high hydrogen yields were observed, suggesting that Fe(110) could be a valid alternative for tar decomposition.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


