Peptide-Based Complex Coacervates Stabilized by Cation−π Interactions for Cell Engineering
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Complex coacervation is a form of liquid–liquid phase separation, whereby two types of macromolecules, usually bearing opposite net charges, self-assemble into dense microdroplets driven by weak molecular interactions. Peptide-based coacervates have recently emerged as promising carriers to deliver large macromolecules (nucleic acids, proteins and complex thereof) inside cells. Thus, it is essential to understand their assembly/disassembly mechanisms at the molecular level in order to tune the thermodynamics of coacervates formation and the kinetics of cargo release upon entering the cell. In this study, we designed histidine-rich peptides consisting of modular sequences in which we systematically incorporate cationic, anionic, or aromatic residues at specific positions along the sequence in order to modulate intermolecular interactions and the resulting coacervation stability. We show that cation−π interactions between arginine and aromatic side chains are particularly efficient in stabilizing complex coacervates, and these interactions can be disrupted in the protein-rich intracellular environment, triggering the disassembly of complex coacervates followed by cargo release. With the additional grafting of a disulfide-based self-immolative side chain, these complex coacervates exhibited enhanced stability and could deliver proteins, mRNA, and CRISPR/Cas9 genome editing tools with tunable release kinetics into cells. This capability extends to challenging cell types, such as macrophages. Our study highlights the critical role of cation−π interactions in the design of peptide-based coacervates, expanding the biomedical and biotechnology potential of this emerging intracellular delivery platform.
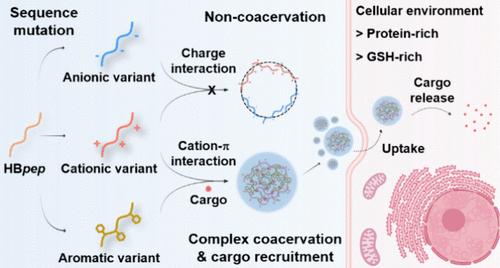
用阳离子- π相互作用稳定肽基络合物凝聚体用于细胞工程
复杂凝聚是一种液-液相分离的形式,两种大分子通常携带相反的净电荷,在弱分子相互作用的驱动下自组装成致密的微滴。以肽为基础的凝聚体最近成为在细胞内递送大分子(核酸、蛋白质及其复合物)的有希望的载体。因此,有必要在分子水平上了解它们的组装/拆卸机制,以便调整凝聚形成的热力学和进入细胞后货物释放的动力学。在这项研究中,我们设计了由模块化序列组成的富含组氨酸的肽,我们系统地在序列的特定位置加入阳离子、阴离子或芳香残基,以调节分子间相互作用和由此产生的凝聚稳定性。我们发现精氨酸和芳香侧链之间的阳离子- π相互作用在稳定复杂凝聚体方面特别有效,并且这些相互作用可以在富含蛋白质的细胞内环境中被破坏,引发复杂凝聚体的分解,随后释放货物。通过附加的基于二硫化物的自牺牲侧链接枝,这些复杂的凝聚体表现出更高的稳定性,可以将蛋白质、mRNA和CRISPR/Cas9基因组编辑工具以可调的释放动力学传递到细胞中。这种能力扩展到挑战细胞类型,如巨噬细胞。我们的研究强调了阳离子- π相互作用在肽基凝聚体设计中的关键作用,扩大了这种新兴细胞内传递平台的生物医学和生物技术潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


