Layered vanadium oxide with tunable layer spacing via dual organic molecule co-insertion for advanced aqueous zinc-ion batteries
IF 6.1
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The narrow layer spacing of layered vanadium pentoxide (α-V2O5) and the slow diffusion kinetics of Zn2+ limit its application in aqueous zinc-ion batteries. The organic molecule pre-insertion strategy has been proposed to improve its electrochemical performance. Nevertheless, the embedding of electrochemically inert organic molecules leads to a decrease in the volume-specific capacity. Here, a bi-organic molecular co-insertion strategy is proposed by introducing electrochemically active cyclohexanehexone octahydrate (KCO) and 1-methyl-2-pyrrolidone (NMP) into α-V2O5 to obtain different spacings of α-V2O5 (11.93, 11.77, 11.06, 8.76, and 7.67 Å) and optimize electrochemical properties by spacing. Furthermore, experiments and DFT calculations reveal that co-insertion provides additional active sites (C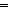 O/C–O), enhances electrical conductivity, and reduces desolvation energy, thus leading to superior zinc storage properties. Thus, benefiting from the collaborative effect of the bi-organic molecules and the suitable layer spacing, VNK4 (8.76 Å) possesses the best electrochemical performance with a 95.7% capacity retention over 100 cycles at 0.5 A g−1 and a specific capacity of up to 190.6 mA h g−1 over 9500 cycles at 10 A g−1. This work offers a novel thought for constructing the organic–inorganic hybrid cathode.
O/C–O), enhances electrical conductivity, and reduces desolvation energy, thus leading to superior zinc storage properties. Thus, benefiting from the collaborative effect of the bi-organic molecules and the suitable layer spacing, VNK4 (8.76 Å) possesses the best electrochemical performance with a 95.7% capacity retention over 100 cycles at 0.5 A g−1 and a specific capacity of up to 190.6 mA h g−1 over 9500 cycles at 10 A g−1. This work offers a novel thought for constructing the organic–inorganic hybrid cathode.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


