EPR Characterization of the BlsE Substrate Radical Offers Insight into the Determinants of Reaction Outcome that Distinguish Radical SAM Dioldehydratases from Dehydrogenases
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
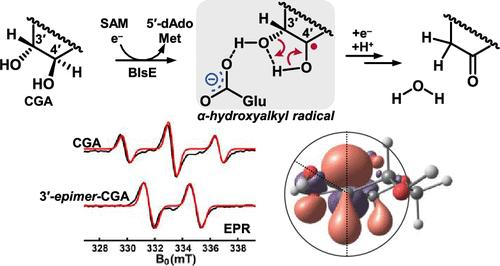
A small but growing set of radical SAM (S-adenosyl-l-methionine) enzymes catalyze the radical mediated dehydration or dehydrogenation of 1,2-diol substrates. In some cases, these activities can be interchanged via minor structural perturbations to the reacting components raising questions regarding the relative importance of hyperconjugation, proton circulation and leaving group stability in determining the reaction outcome. The present work describes trapping and electron paramagnetic resonance (EPR) characterization of an α-hydroxyalkyl radical intermediate during dehydration and dehydrogenation of cytosylglucuronic acid and its derivatives catalyzed by the radical SAM enzyme BlsE and its Glu189Ala mutant from the blasticidin S biosynthetic pathway. The substrate radical is found to have a dihedral angle between the electron spin carrier p-orbital and the C–O bond to be cleaved that appears to be sufficient to support elimination despite lying outside the strictly periplanar region. A more significant contributor to the gating of dehydration activity, however, appears to be establishment of a proper hydrogen bonding configuration in order to stabilize the accumulation of negative charge on the eliminated hydroxyl group.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


