A Second Near-Infrared Window-Responsive Metal–Organic-Framework-Based Photosensitizer for Tumor Immunotherapy via Synergistic Ferroptosis and STING Activation
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
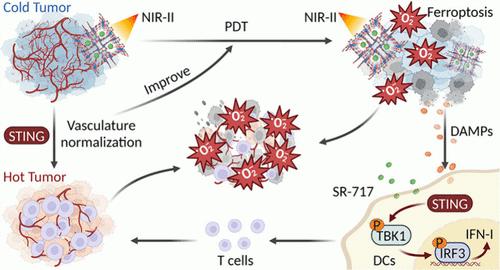
Photodynamic therapy (PDT) holds promise as a cancer treatment modality due to its potential for enhanced therapy precision and safety. To enhance deep tissue penetration and minimize tissue adsorption and phototoxicity, developing photosensitizers activated by second near-infrared window (NIR-II) light shows significant potential. However, the efficacy of PDT is often impeded by tumor microenvironment hypoxia, primarily caused by irregular tumor vasculature. Fortunately, the stimulator of interferon genes (STING) pathway, known for immune activation, has been linked to vasculature normalization. In this study, we developed a nanoplatform (Fe-THBQ/SR) by loading a STING agonist (SR-717) into an iron-tetrahydroxy-1,4-benzoquinone (Fe-THBQ) metal–organic framework. Fe-THBQ was proven to be an effective NIR-II photosensitizer, generating numerous reactive oxygen species (ROS) under 1064 nm laser irradiation. These ROS downregulated heat shock protein expression, consequently promoting mild-photothermal therapy (mild-PTT), and facilitated ferroptosis by depleting glutathione (GSH)/glutathione peroxidase 4. Moreover, Fe-THBQ/SR released SR-717 upon GSH stimulation, synergizing with the ROS-mediated double-stranded DNA leakage to enhance STING activation. This process contributed to tumor vasculature normalization and hypoxia alleviation, thereby enhancing the PDT efficacy. Overall, we presented a versatile single-laser-triggered nanoplatform (Fe-THBQ/SR) for NIR-II PDT and NIR-II mild-PTT and simultaneously coupled it with the effective activation of STING to form a reinforcing cycle. These synergistic enhancements increased the immunogenicity of tumor cells, remodeled the immunosuppressive tumor microenvironment, increased T lymphocyte infiltration, and improved therapeutic outcomes.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


