Operando Characterization of Fe in Doped Nix(Fe1–x)OyHz Catalysts for Electrochemical Oxygen Evolution
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
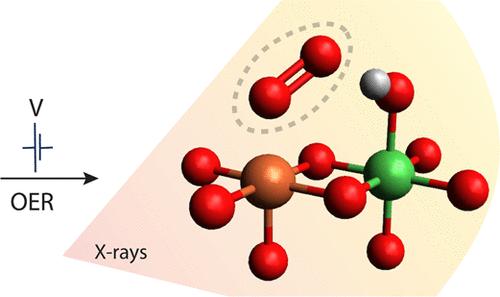
Iron-doped nickel oxyhydroxides, Nix(Fe1–x)OyHz, are among the most promising oxygen evolution reaction (OER) electrocatalysts in alkaline environments. Although iron (Fe) significantly enhances the catalytic activity, there is still no clear consensus on whether Fe directly participates in the reaction or merely acts as a promoter. To elucidate the Fe’s role, we performed operando X-ray spectroscopy studies supported by DFT on Nix(Fe1–x)OyHz electrocatalysts. We probed the reversible changes in the structure and electronic character of Nix(Fe1–x)OyHz as the electrode potential is cycled between the resting (here at 1.10 VRHE) and operational states (1.66 VRHE). DFT calculations and XAS simulations on a library of Fe structures in various NiOyHz environments are in favor of a distorted local octahedral Fe(III)O3(OH)3 configuration at the resting state with the NiOyHz scaffold going from α-Ni(OH)2 to γ-NiOOH as the potential is increased. Under catalytic conditions, EXAFS and HERFD spectra reveal changes in p-d mixing (covalency) relative to the resting state between O/OH ligands and Fe leading to a shift from octahedral to square pyramidal coordination at the Fe site. XES measurements and theoretical simulations further support that the Fe equilibrium structure remains in a formal Fe(III) state under both resting and operational conditions. These spectral changes are attributed to potential dependent structural rearrangements around Fe. The results suggest that ligand dissociation leads to the C4v symmetry as the most stable intermediate of the Fe during OER. This implies that Fe has a weakly coordinated or easily dissociable ligand that could serve to coordinate the O–O bond formation and, tentatively, play an active role in the Nix(Fe1–x)OyHz electrocatalyst.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


