Glial activation and nociceptive neuropeptide elevation associated with the development of chronic post-traumatic headache following repetitive blast exposure
Q2 Medicine
引用次数: 0
Abstract
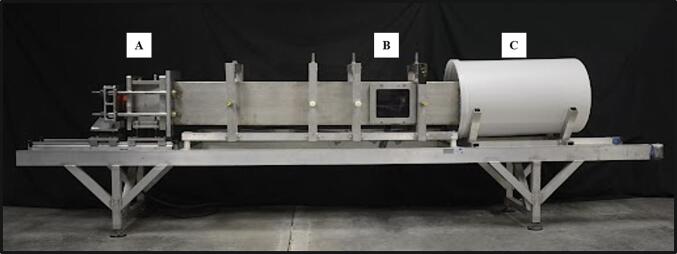
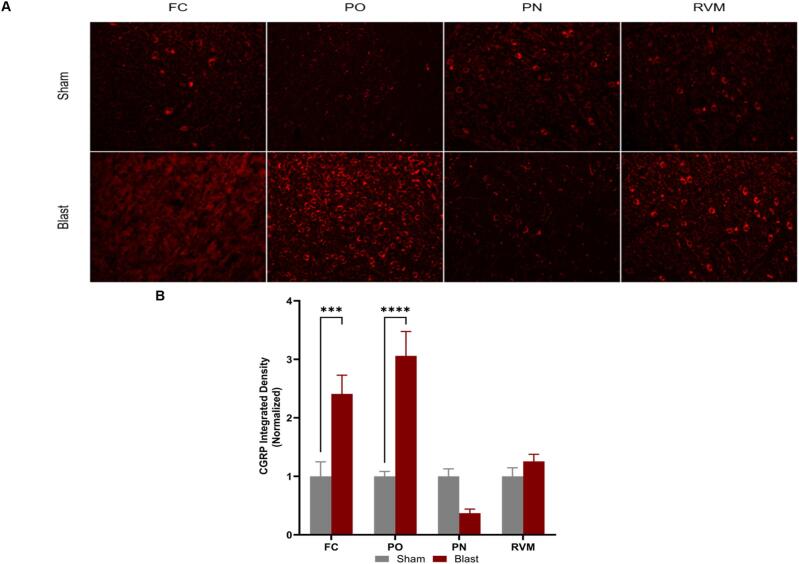
Chronic headaches and pain are prevalent in those who are exposure to blast events, yet there is a gap in fundamental data that identifies the pathological mechanism for the chronification of pain. Blast-related post-traumatic headaches (PTH) are understudied and chronic pain behaviors in preclinical models can be vital to help elucidate PTH mechanisms. The descending pain modulatory system controls pain perception and involves specific brain regions such as the cortex, thalamus, pons, and medulla. In this study, male rats were exposed to repeated blast events to induce traumatic brain injury (bTBI) and subsequently assessed for the development of PTH by testing for chronic pain behaviors and examining the neuropathology of the descending pain pathway. The results demonstrated that facial hypersensitivity developed as early as week two following bTBI and persisted throughout the study (12 weeks). Depressive-like behaviors were observed at 12 weeks following bTBI, and these behaviors were associated with neuropathologies such as microglia ramification and neuropeptide elevation (Calcitonin Gene-Related Peptide, CGRP; Substance P, SP). Overall, these findings support the hypothesis that bTBI causes the activation of microglia and elevation of neuropeptides, which contribute to the development of chronic PTH behaviors.
神经胶质细胞激活和伤害神经肽升高与反复爆炸暴露后慢性创伤后头痛的发展有关。
慢性头痛和疼痛在暴露于爆炸事件的人群中普遍存在,但在确定疼痛慢性化的病理机制的基础数据方面存在空白。爆炸相关的创伤后头痛(PTH)尚未得到充分研究,临床前模型中的慢性疼痛行为对阐明PTH机制至关重要。下行疼痛调节系统控制疼痛感知,涉及特定的大脑区域,如皮层、丘脑、脑桥和髓质。在这项研究中,雄性大鼠暴露于重复爆炸事件诱导创伤性脑损伤(bTBI),随后通过检测慢性疼痛行为和检查下行疼痛通路的神经病理学来评估PTH的发展。结果表明,面部过敏早在脑外伤后第2周就出现,并持续整个研究(12周)。在bTBI后12周观察到抑郁样行为,这些行为与神经病理相关,如小胶质细胞分支和神经肽升高(降钙素基因相关肽,CGRP;物质P, SP)。总的来说,这些发现支持了bTBI引起小胶质细胞激活和神经肽升高的假设,这有助于慢性PTH行为的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurobiology of Pain
Medicine-Anesthesiology and Pain Medicine
CiteScore
4.40
自引率
0.00%
发文量
29
审稿时长
54 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


