Synergistic Enhancement of Ferroptosis via Mitochondrial Accumulation and Photodynamic-Controlled Release of an Organogold(I) Cluster Prodrug
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Effective delivery and controlled release of metallo-prodrugs with sustained activation and rapid response feed the needs of precise medicine in metal chemotherapeutics. However, gold-based anticancer drugs often suffer from detoxification binding and extracellular transfer by sulfur-containing peptides. To address this challenge, we integrate a thiol-activated prodrug strategy of newly prepared hypercoordinated carbon-centered gold(I) clusters (HCGCs) with their photosensitization character to augment the mitochondrial release of Au(I) in tumors. In contrast to the distorted [CAu4] kernel of a pentanuclear HCGC compound [A5], its dimeric congener [A9] exhibits a symmetrical [{CAu4}–Au–{CAu4}] structure with a remarkable hypercarbon-to-Au4 electron donation. This unique arrangement results in a microsecond long metal–metal-to-ligand charge transfer excited state relative to the nanosecond intraligand excited state of [A5]. Upon light irradiation at 560 nm, [A9] generates active 1O2 to oxidize glutathione (GSH) into poorly coordinating GSSG in the cytoplasm and finally promotes subcellular delivery of HCGCs to mitochondria. Moreover, GSH further triggers consecutive release of active [AuPPh3]+ ions to inhibit cytoplasmic glutathione peroxidase GPX4 and mitochondrial thioredoxin reductase TrxR2, which collectively result in accelerated ferroptosis of human bladder cancer EJ cells and show excellent antitumor performance in mouse bladder tumor models.
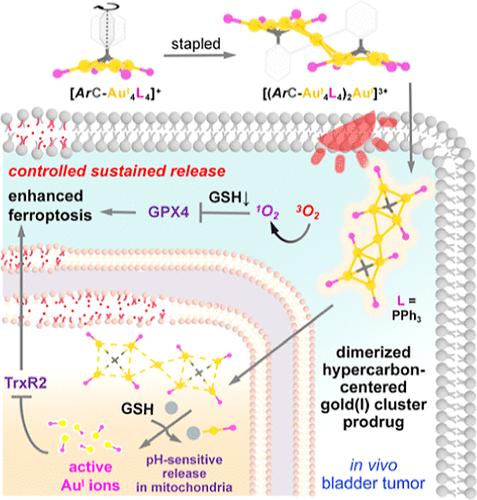
一种有机金(I)簇前药通过线粒体积累和光动力控制释放协同增强铁下垂
金属前药的有效递送和控释、持续活化和快速反应满足了金属化疗精准用药的需要。然而,含硫肽对含金抗肿瘤药物的解毒作用和细胞外转移往往是金类抗肿瘤药物的主要缺陷。为了解决这一挑战,我们将新制备的超协调碳中心金(I)簇(HCGCs)的硫醇激活前药策略与其光敏特性结合起来,以增加肿瘤中金(I)的线粒体释放。与五核HCGC化合物[A5]的扭曲[CAu4]核不同,其二聚体同族化合物[A9]呈现出对称的[{CAu4} - au - {CAu4}]结构,并具有显著的超碳- au4电子赋能。相对于[A5]的纳秒配体内激发态,这种独特的排列导致了微秒长的金属-金属到配体的电荷转移激发态。在560nm光照射下,[A9]产生活性1O2,将谷胱甘肽(GSH)氧化为细胞质中不协调的GSSG,最终促进HCGCs向线粒体的亚细胞递送。GSH进一步触发活性[AuPPh3]+离子的连续释放,抑制细胞质谷胱甘肽过氧化物酶GPX4和线粒体硫氧还蛋白还原酶TrxR2,共同加速人膀胱癌EJ细胞的铁凋亡,并在小鼠膀胱肿瘤模型中表现出优异的抗肿瘤性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


