Unveiling Heavier Dihydropyridine Chalcogenol Esters in Metallaphotoredox Catalyst-Enabled Regioselective Hydrothio(seleno)carbonylation
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, aromaticity-driven thio(seleno)ester group transfer from novel 1,4-dihydropyridine thio(seleno)esters to alkene feedstocks is disclosed by merging palladium and photoredox catalysis. In this process, photoactivation of dihydropyridine thio(seleno)esters is integrated with regioselective hydrometalation of alkenes, avoiding photoinduced Pd–C bond homolysis of organopalladium intermediates. Additionally, a regioselective hydroselenocarbonylation of an alkene is accomplished for the first time using a bench-stable selenoester reagent. The activation mode of novel dihydropyridine thioesters has been illustrated by detailed mechanistic studies, spectroscopic analysis, intermediate trapping, and isotope labeling experiments.
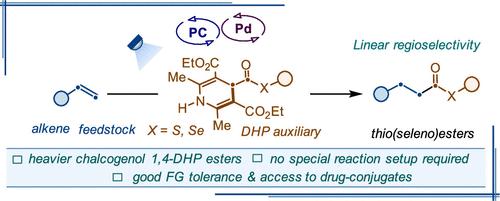
揭示金属光氧化还原催化区域选择性氢硫(硒)羰基化中较重的二氢吡啶硫醇酯
本文公开了一种新型1,4-二氢吡啶硫代(硒代)酯通过合并钯和光氧化还原催化将芳香族驱动的硫代(硒代)酯基团从硫代(硒代)酯转移到烯烃原料。在此过程中,二氢吡啶硫代(硒)酯的光活化与烯烃的区域选择性金属加氢化相结合,避免了光诱导的有机钯中间体的Pd-C键均裂。此外,本发明首次使用稳定的硒酸酯试剂实现了烯烃的区域选择性氢硒羰基化。通过详细的机理研究、光谱分析、中间俘获和同位素标记实验,阐明了新型二氢吡啶硫酯的活化模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


