Cytoplasmic mRNA decay controlling inflammatory gene expression is determined by pre-mRNA fate decision
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
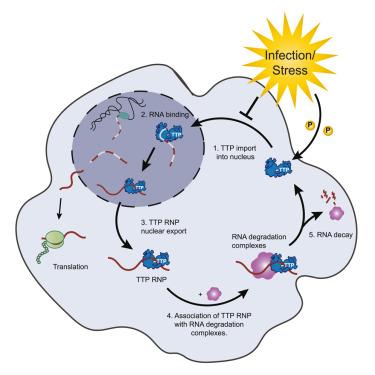
The fidelity of immune responses depends on timely controlled and selective mRNA degradation that is largely driven by RNA-binding proteins (RBPs). It remains unclear whether stochastic or directed processes govern the selection of an individual mRNA molecule for degradation. Using human and mouse cells, we show that tristetraprolin (TTP, also known as ZFP36), an essential anti-inflammatory RBP, destabilizes target mRNAs via a hierarchical molecular assembly. The assembly formation strictly relies on the interaction of TTP with RNA. The TTP homolog ZFP36L1 exhibits similar requirements, indicating a broader relevance of this regulatory program. Unexpectedly, the assembly of the cytoplasmic mRNA-destabilization complex is licensed in the nucleus by TTP binding to pre-mRNA, which we identify as the principal TTP target rather than mRNA. Hence, the fate of an inflammation-induced mRNA is decided concomitantly with its synthesis. This mechanism prevents the translation of excessive and potentially harmful inflammation mediators, irrespective of transcription.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


