Proton-Controlled Electron Injection in MoS2 During Hydrogen Evolution Revealed by Time-Resolved Spectroelectrochemistry
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
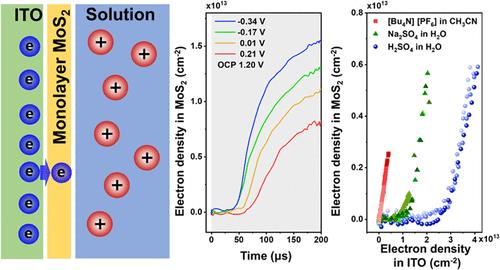
Monolayer MoS2 is an effective electrocatalyst for the hydrogen evolution reaction (HER). Despite significant efforts to optimize the active sites, its catalytic performance still falls short of theoretical predictions. One key factor that has often been overlooked is the electron injection from the conductive substrate into the MoS2. The charge transfer behavior at the substrate-MoS2 interface is nonclassical, exhibiting a liquid-gated electron injection behavior, the underlying mechanism of which remain under debate. To investigate this, we employ nanosecond time-resolved spectroelectrochemistry to probe the electron injection dynamics into monolayer MoS2 under operando HER conditions. Simultaneously, transient current measurements provide insights into the electron density at the substrate. By combining the electron density obtained from the MoS2 through spectroelectrochemical analysis with the electron density at the conductive substrate derived from transient current measurements, we explore the electron injection dynamics and characterize the current density potential (J-E) behavior at the substrate-MoS2 interface. Our findings show that the electron injection barrier and capability correlate strongly with proton concentration in the electrolyte. This relationship likely reflects the electron concentration-dependent conductivity of MoS2, where higher proton concentrations lead to fewer stray electrons before injection begins.
时间分辨光谱电化学研究MoS2中质子控制电子注入的析氢过程
单层二硫化钼是一种有效的析氢反应电催化剂。尽管对活性位点进行了大量的优化,但其催化性能仍低于理论预测。一个经常被忽视的关键因素是电子从导电衬底注入二硫化钼。衬底-二硫化钼界面处的电荷转移行为是非经典的,表现为液体门控电子注入行为,其潜在机制仍存在争议。为了研究这一点,我们采用纳秒时间分辨光谱电化学技术来探测在操作HER条件下单层二硫化钼的电子注入动力学。同时,瞬态电流测量提供了对衬底电子密度的深入了解。通过将光谱电化学分析得到的MoS2的电子密度与瞬态电流测量得到的导电衬底处的电子密度相结合,我们探索了电子注入动力学,并表征了衬底-MoS2界面处的电流密度势(J-E)行为。我们的研究结果表明,电子注入势垒和能力与电解质中的质子浓度密切相关。这种关系可能反映了MoS2的电子浓度依赖电导率,其中较高的质子浓度导致在注入开始前较少的杂散电子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


