Fully Atomistic Molecular Dynamics Simulation of Ice Nucleation Near an Antifreeze Protein
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Heterogeneous ice nucleation is a widespread phenomenon in nature. Despite extensive research on ice nucleation near biological antifreeze proteins, a probe for ice nucleation and growth processes at the atomic level is still lacking. Herein, we present simulation evidence of the heterogeneous ice nucleation process on the ice-binding surface (IBS) of the Tenebrio molitor antifreeze protein (TmAFP). Our all-atomistic molecular dynamics simulations reveal detailed steps toward precritical nucleus formation from one-dimensional (1D) channel water to a 2D ice nanolayer and, finally, a 3D ice nucleus. Compared with homogeneous ice nucleation under the same supercooling conditions, the IBS of TmAFP can markedly reduce the critical size of the ice embryo and lower the nucleation free energy barrier, thereby favoring ice nucleation. Additionally, through artificial mutation of selected functional groups on the IBS, we gain deeper insights into how the specific functional groups of the IBS affect ice nucleation. We highlight that the carbonyl groups in the backbone play a crucial role by providing fixed locations for channel water. This function is essential for ensuring alignment between the 2D ice nanolayer and the ice lattice structure.
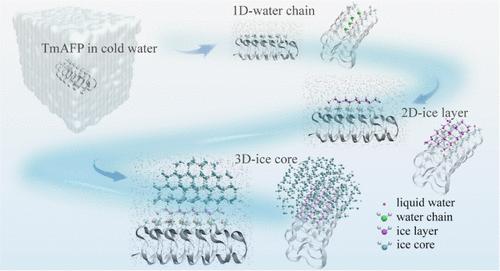
抗冻蛋白附近冰核的全原子分子动力学模拟
非均相冰成核是自然界普遍存在的现象。尽管对生物抗冻蛋白附近的冰成核进行了广泛的研究,但在原子水平上对冰成核和生长过程的探测仍然缺乏。在此,我们提供了在黄粉虫抗冻蛋白(TmAFP)冰结合表面(IBS)上非均质冰核过程的模拟证据。我们的全原子分子动力学模拟揭示了从一维通道水到二维冰纳米层,最后到三维冰核形成临界核的详细步骤。与相同过冷条件下的均匀冰形核相比,TmAFP的IBS可以显著降低冰胚的临界尺寸,降低成核自由能垒,有利于冰形核。此外,通过人工突变IBS上的特定官能团,我们更深入地了解了IBS的特定官能团如何影响冰成核。我们强调主链中的羰基通过为通道水提供固定位置起着至关重要的作用。这个功能对于确保二维冰纳米层和冰格结构之间的对齐是必不可少的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


