Neoadjuvant immunotherapy for non-small cell lung cancer: Opportunities and challenges
Chinese medical journal pulmonary and critical care medicine
Pub Date : 2024-12-01
DOI:10.1016/j.pccm.2024.11.003
引用次数: 0
Abstract
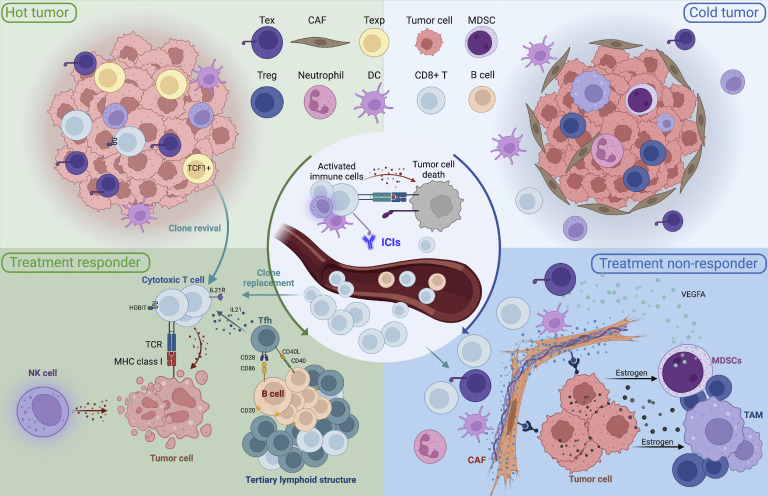
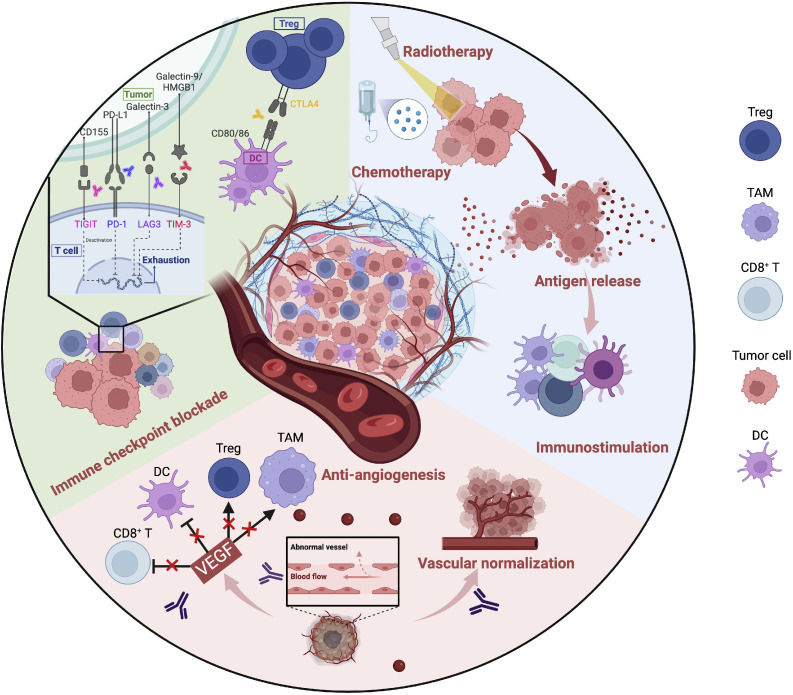
Immune checkpoint inhibitors (ICIs) have transformed the treatment landscape for resectable non-small cell lung cancer. Numerous trials have explored the use of ICIs, either as monotherapy or in combination with other therapies, in the neoadjuvant setting for stage I–III non-small cell lung cancer. Most trials have demonstrated neoadjuvant immunotherapy to be safe and to have remarkable efficacy, with a high pathological response rate and significantly improved event-free survival. This review summarizes the findings of Phase I–III clinical trials investigating various neoadjuvant regimens, including ICI monotherapy, ICI therapy combined with chemotherapy, ICI plus anti-angiogenic therapy, dual ICI therapy, and ICI therapy in combination with radiotherapy or chemoradiotherapy. We discuss the benefits and outcomes associated with each approach. Despite the results being promising, several unresolved issues remain, including identification of reliable biomarkers, the appropriate duration of therapy, the optimal treatment regimen for tumors with high programmed cell death ligand 1 (PD-L1) expression, the false-negative pathological complete response rate, and the role of digital pathology in assessing the response to treatment. Resistance to immunotherapy, in particular, remains a significant barrier to effective use of ICIs. Given the critical influence of the tumor microenvironment (TME) on the response to treatment, we examine the characteristics of the TME in both responsive and resistant tumors as well as the dynamic changes that occur in the TME in response to neoadjuvant immunotherapy. We also summarize the mechanisms underlying T cell responses following neoadjuvant immunotherapy and provide a perspective on strategies to enhance the understanding of tumor heterogeneity, therapy-driven TME remodeling, and overcoming resistance to therapy. Finally, we propose future directions for advancements in personalized neoadjuvant immunotherapy.
非小细胞肺癌的新辅助免疫治疗:机遇与挑战。
免疫检查点抑制剂(ICIs)已经改变了可切除非小细胞肺癌的治疗前景。许多试验已经探索了在I-III期非小细胞肺癌的新辅助治疗中使用ICIs,无论是作为单一疗法还是与其他疗法联合使用。大多数试验表明,新辅助免疫治疗是安全的,疗效显著,病理反应率高,无事件生存率显著提高。本文综述了研究各种新辅助治疗方案的I-III期临床试验的结果,包括ICI单药治疗、ICI联合化疗、ICI加抗血管生成治疗、双重ICI治疗、ICI联合放疗或放化疗。我们将讨论与每种方法相关的好处和结果。尽管结果很有希望,但仍存在一些未解决的问题,包括确定可靠的生物标志物,适当的治疗时间,高程序性细胞死亡配体1 (PD-L1)表达的肿瘤的最佳治疗方案,假阴性病理完全缓解率,以及数字病理学在评估治疗反应中的作用。特别是对免疫疗法的耐药性,仍然是有效使用免疫球蛋白的一个重大障碍。鉴于肿瘤微环境(TME)对治疗反应的关键影响,我们研究了反应性和耐药肿瘤中TME的特征,以及新辅助免疫治疗后TME发生的动态变化。我们还总结了新辅助免疫治疗后T细胞反应的机制,并提供了增强对肿瘤异质性、治疗驱动的TME重塑和克服治疗耐药性的策略的观点。最后,我们提出了个性化新辅助免疫治疗的未来发展方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese medical journal pulmonary and critical care medicine
Critical Care and Intensive Care Medicine, Infectious Diseases, Pulmonary and Respiratory Medicine
CiteScore
0.40
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


