Effect of Doping on Electrocatalytic Dehydrogenation and Hydrogenation of Methyl Decalin–Methyl Naphthalene System
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The hydrogen economy can benefit from the use of liquid organic hydrogen carriers (LOHCs) for cross-continent hydrogen transportation in the future. However, dehydrogenation and hydrogenation of hydrogen require catalytic systems. Current research emphasizes selective Pt/Rh doping of Fe, Co, and Ni surfaces as catalysts for the dehydrogenation and hydrogenation of the methyl naphthalene–methyl decalin LOHC system, which has more than 7% hydrogen weight capacity and meets the practical requirements established by the European Union and the United States Department of Energy. Density functional theory-based computational techniques demonstrate how the chemical modification of these surfaces with a Pt and Rh single-atom catalyst (SAC) can improve the efficiency of dehydrogenation and hydrogenation. With a sustainable method, electrochemical dehydrogenation and hydrogenation on these robust surfaces produce effective hydrogen storage for extended periods without losing hydrogen. Furthermore, optimal results for the hydrogenation of 2-methyl naphthalene on Fe–Rh SAC with path-determining step (PDS) = 0.98 eV and dehydrogenation of 1-methyl decalin on Fe–Pt SAC with PDS = 1.49 eV were obtained for the most effective active sites for the enhanced electrochemical process. This study offers new possibilities for the catalytic dehydrogenation and hydrogenation of LOHC systems by highlighting the impact of doping on transition-metal-based catalysts.
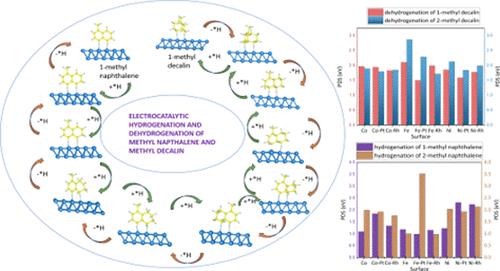
掺杂对十余年甲基萘-甲基萘体系电催化脱氢和加氢的影响
氢经济可以从使用液态有机氢载体(lohc)进行跨大陆氢运输中受益。然而,氢的脱氢和加氢需要催化系统。目前的研究重点是在Fe、Co和Ni表面选择性掺杂Pt/Rh,作为萘甲基-十氢化萘甲基LOHC体系脱氢和加氢的催化剂,该体系氢重容量大于7%,符合欧盟和美国能源部制定的实际要求。基于密度泛函理论的计算技术证明了用Pt和Rh单原子催化剂(SAC)对这些表面进行化学修饰可以提高脱氢和加氢效率。通过一种可持续的方法,在这些坚固的表面上进行电化学脱氢和加氢,可以在不损失氢的情况下长时间有效地储存氢。结果表明,2-甲基萘在Fe-Rh SAC上加氢(PDS = 0.98 eV)和1-甲基萘在Fe-Pt SAC上脱氢(PDS = 1.49 eV)是强化电化学过程最有效的活性位点。本研究通过突出掺杂对过渡金属基催化剂的影响,为LOHC体系的催化脱氢和加氢提供了新的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


