Hyperconjugation Engineering of π-Extended Azaphosphinines for Designing Tunable Thermally Activated Delayed Fluorescence Emitters
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Implanting heteroatoms into organic π-conjugated molecules (OCMS) offered a great opportunity to fine-tune the chemical structures and optoelectronic properties. This work describes a new family of 1,4-azaphosphinines with extended σ–π hyperconjugations. The photophysical studies revealed that azaphosphinines exhibited narrow-band thermally activated delayed fluorescence (TADF) ( full width at half-maximum: 26–40 nm). According to the orbital localization analysis and natural bond orbital analysis, the effective σ*−π* hyperconjugation is believed to induce the multiple-resonance (MR) TADF, which is distinct from the p−π conjugation-induced MR-TADF in BN systems. Although having the large ΔES1–T1s (>3.0 ev), the study suggested that σ*−π hyperconjugation endowed the system with the structural vibration favorable for the spin-vibronic-assisted RISC. Having the tunable p-centers (lp, O, S, Se, and Me+), azaphosphinines showed a fine-tuned TADF. Generally, azaphosphinines with strong σ*−π* hyperconjugations showed small ΔES1–T1s, efficient RISCs, and high PLQYs. Leveraging on the efficient hyperconjugations, TADF emission of the system spanned from UV-blue to green. Particularly, extended azaphosphinines exhibited the high photoluminescence quantum yields (74% in toluene and 92% in the 10% doped PMMA). As a proof of concept, two azaphosphinines with a PO center were applied as the light-emitting materials in organic lighting-emitting diodes. The devices showed the narrow-band UV- and deep-blue emission with EQE as high as 10.3%. The current study offered us a new strategy, namely, σ–π hyperconjugation-induced MR-TADF, for designing OCMs with tunable light-emitting properties.
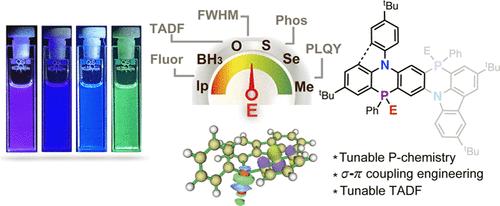
π-扩展氮杂膦的超共轭工程设计可调谐热激活延迟荧光发射器
在有机π共轭分子(OCMS)中植入杂原子为精细调整其化学结构和光电子性质提供了良好的机会。本文描述了一个新的具有扩展σ -π超共轭的1,4-氮杂膦族。光物理研究表明,氮磷碱表现出窄带热激活延迟荧光(TADF)(半最大值全宽度:26-40 nm)。根据轨道定位分析和自然键轨道分析,认为有效σ*−π*超共轭可以诱导多共振(MR) TADF,而不同于BN体系中p−π共轭诱导的MR-TADF。虽然具有较大的ΔES1-T1s (>3.0 ev),但研究表明σ*−π超共轭使体系具有有利于自旋振动辅助RISC的结构振动。由于具有可调的p中心(lp、O、S、Se和Me+),氮磷碱具有可调的TADF。一般来说,具有强σ*−π*超共轭的氮扎膦具有小ΔES1-T1s,高效的RISCs和高PLQYs。利用高效的超共轭,系统的TADF发射范围从紫外蓝到绿色。特别地,扩展型氮磷啉表现出较高的光致发光量子产率(在甲苯中为74%,在掺10% PMMA中为92%)。作为概念验证,两个具有PO中心的氮氮膦作为发光材料应用于有机发光二极管。器件显示出窄带紫外和深蓝发射,EQE高达10.3%。本研究为设计具有可调谐发光特性的光器件提供了一种新的策略,即σ -π超共轭诱导的MR-TADF。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


