Orbital Resolution of the Reconstruction of CeO2 (100) Facet–Hybrid-DFT and COHP Investigations Supported by HR-TEM Imaging
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
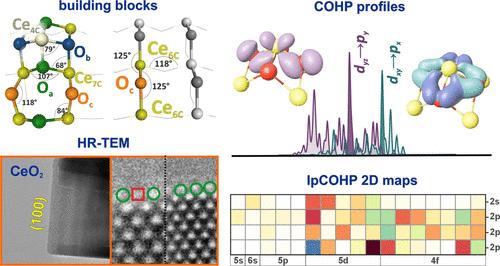
A detailed understanding of the atomic-level structure and reconstruction of the (100) facet of ceria is crucial for optimizing its performance in various thermo-, photo-, and electrocatalytic processes. Herein, the geometric and molecular orbital structures of the stoichiometric Ohalf-t (half terminated with O), Cehalf-t (half terminated with Ce), and CeO4-t (terminated with CeO4 pyramids) terminations of the (100) ceria surface were thoroughly investigated using periodic (HSE06 and PW91+U DFT) calculations and first-principles thermodynamic modeling, complemented by an extended COHP analysis. The structure and the stability of these terminations were analyzed, for the first time, in terms of three generic building blocks, including bridging (b-Ce2O), square pyramidal (py-CeO4), and tandem (py-CeO4∩b-Ce2O) units and characterized with molecular orbital resolution. The atomic-wise COHP profiles were decomposed into the orbital-wise interactions within the building units, and the associated overlaps between the 4f;5s,p,d;6s-Ce and 2s,p-O orbitals were presented in the form of 2D maps with the IpCOHP values gauging their strengths. The key bonds and orbital interactions responsible for the stability of the particular building block were identified, providing a detailed molecular rationale for the thermodynamic stability order of the CeO4-t (1.16 J·m–2) ∼ Ohalf-t (1.21 J·m–2) > Cehalf-t (1.32 J·m–2) terminations. The predicted coexistence of the CeO4-t and Ohalf-t terminations was finally confirmed by HR-TEM imaging of euhedral CeO2 nanocubes supported by image simulations. Ceria nanocubes were synthesized by the hydrothermal method without surfactants.
基于HR-TEM的CeO2 (100) Facet-Hybrid-DFT和COHP重建的轨道分辨率
详细了解铈的原子级结构和(100)面重构对于优化其在各种热、光和电催化过程中的性能至关重要。本文采用周期(HSE06和PW91+U DFT)计算和第一线热力学模型,结合扩展的COHP分析,全面研究了(100)铈表面的Ohalf-t(一半末端为O)、Cehalf-t(一半末端为Ce)和CeO4-t(末端末端为CeO4金字塔)的化学计量结构的几何和分子轨道结构。首次用桥接(b-Ce2O)、方锥体(py-CeO4)和串联(py-CeO4∩b-Ce2O)三个基本单元分析了这些末端的结构和稳定性,并用分子轨道分辨率进行了表征。将原子方向的COHP曲线分解为建筑单元内轨道方向的相互作用,并将4f;5s,p,d;6s-Ce和2s,p- o轨道之间的相关重叠以2D图的形式呈现,并用IpCOHP值衡量它们的强度。确定了特定构建块稳定性的关键键和轨道相互作用,为CeO4-t (1.16 J·m-2) ~ Ohalf-t (1.21 J·m-2) >的热力学稳定性顺序提供了详细的分子理论基础;Cehalf-t (1.32 J·m-2)终止。通过对自面体CeO2纳米立方体的HR-TEM成像和图像模拟证实了预测的CeO4-t和Ohalf-t末端共存。采用水热法合成了氧化铈纳米立方。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


