Modular Synthesis of Planar-Chiral Cyclononenes via trans-Retentive Trapping of π-Allyl-Pd Dipoles
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Trans-cycloalkenes are abundant in bioactive natural products and have been used as powerful tools in chemical biology and drug discovery. However, strategies for the modular synthesis of trans-cycloalkenes, especially planar-chiral medium-sized ones, with high efficiency and selectivity, still remain elusive. Herein, we report a Pd-catalyzed asymmetric [7 + 2] cyclization strategy to address this challenge. As a result, two types of planar-chiral trans-cyclononenes bearing all-carbon chiral quaternary stereocenters are produced in up to 85% yield, >99% ee, and >19:1 dr (42 examples). The key to this success is the maintenance of the trans-2H configuration of the π-allyl-Pd species along with unusual linear selectivity during the H-bond-assisted cyclization process. In addition, the conversion of planar chirality to central chirality and its application in selective bioimaging of cancer cells via a bioorthogonal reaction were performed to demonstrate the synthetic value of this methodology.
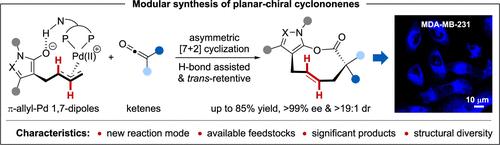
利用π-烯丙基-钯偶极子的反式保留俘获模合成平面手性环壬烯
反式环烯烃是一种丰富的具有生物活性的天然产物,已成为化学生物学和药物发现的有力工具。然而,高效、高选择性地模块化合成反式环烯烃,特别是平面手性中等尺寸的反式环烯烃的方法仍是一个谜。在此,我们报告了一种pd催化的不对称[7 + 2]环化策略来解决这一挑战。结果,两种含全碳手性季位立体中心的平面手性反环杂环烯的产率高达85%,分别为99% ee和19:1 dr(42个例子)。这一成功的关键是在氢键辅助环化过程中维持π-烯丙基- pd的反式2h构型以及异常的线性选择性。此外,通过生物正交反应将平面手性转化为中心手性,并将其应用于癌细胞的选择性生物成像,以证明该方法的合成价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


