A Protein Cleavage Platform Based on Selective Formylation at Cysteine Residues
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Site-selective cleavage of the peptide backbone in proteins is an important class of post-translational modification (PTM) in nature. However, the organic chemistry for such site-selective peptide bond cleavages has yet to be fully explored. Herein, we report cysteine S-formylation as a means of selective protein backbone cleavage. We developed N-formyl sulfonylanilide as a cysteine-selective formylation reagent for peptides and proteins. Upon S-formylation with the reagent, the amide bond adjacent to the S-formylated cysteine is cleaved by hydrolysis under neutral aqueous conditions. Formylation probes bearing a protein ligand enabled the affinity-based selective cleavage of the target proteins not only in the test tube but also under biorelevant conditions such as in crude cell lysate and on the cell surface. These results demonstrate the high biocompatibility of this protein cleavage technology. A proof-of-concept study of cleavage-induced protein activation further demonstrates its utility as a platform for the functional regulation of proteins by artificial PTM.
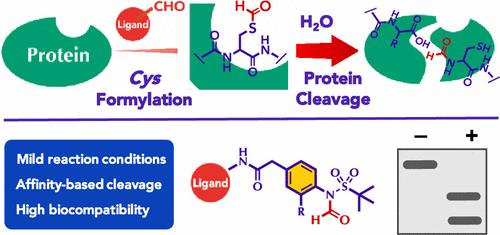
基于半胱氨酸残基选择性甲酰化的蛋白质切割平台
蛋白质中肽骨架的位点选择性切割是一类重要的翻译后修饰(PTM)。然而,这种位点选择性肽键裂解的有机化学尚未得到充分的探索。在这里,我们报告了半胱氨酸s -甲酰化作为选择性蛋白质骨干切割的一种手段。我们开发了n -甲酰基磺酰苯胺作为半胱氨酸选择性的多肽和蛋白质甲酰化试剂。用该试剂进行s -甲酰化后,在中性水条件下,与s -甲酰化半胱氨酸相邻的酰胺键被水解切断。携带蛋白质配体的甲酰化探针不仅可以在试管中,而且可以在生物相关条件下(如粗细胞裂解液和细胞表面)对靶蛋白进行基于亲和的选择性切割。结果表明,该蛋白切割技术具有较高的生物相容性。一项关于卵裂诱导的蛋白质激活的概念验证研究进一步证明了它作为人工PTM对蛋白质功能调节的平台的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


