Divergent application of 5-amino-isoxazoles for the construction of nitrogen heterocycles via the hydride transfer strategy†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-01-22
DOI:10.1039/d4qo02059f
引用次数: 0
Abstract
The hydride transfer-enabled divergent application of 5-amino-isoxazoles for the controllable construction of diverse tetrahydroquinolines and tetrahydroquinazolines was disclosed unprecedentedly by the process of ring-cleavage/Beckmann rearrangement, dearomative-spirocyclization, or N,N′-dialkylation of the amino group with the employment of different Lewis acids.

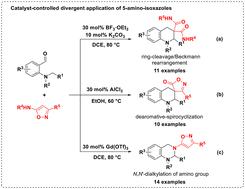
5-氨基异恶唑在氢化物转移策略下构建氮杂环中的不同应用
利用不同的Lewis酸对氨基进行解环/贝克曼重排、脱芳-螺旋环化或N,N ' -二烷基化,从而实现了5-氨基异唑在不同类型四氢喹啉和四氢喹唑类化合物中氢化物转移的分散应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


