Sc-Catalyzed Asymmetric [2 + 2] Annulation of 2-Alkynylnaphthols with Dienes to Access Cyclobutene Frameworks
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we introduce a scandium-catalyzed synthetic strategy that provides access to a diverse and functionalized array of cyclobutene frameworks adorned with a quaternary carbon center. This approach broadens the synthetic repertoire of 2-alkynylnaphthols with alkenes, offering a versatile platform for the construction of complex molecular architectures. The asymmetric catalytic [2 + 2] cycloaddition reaction demonstrates a wide substrate scope and an impressive functional group tolerance, yielding products with high efficiency, up to 97% yield, and excellent enantiomeric excess of up to 97%. The simplicity of scaling up this process, coupled with the ease of converting these cyclobutene frameworks into a variety of substituted products, significantly enhances the synthetic utility of this method.
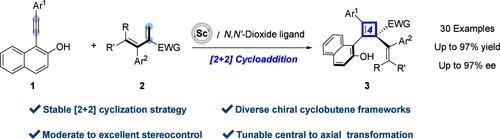
sc催化2-炔基萘酚与二烯的不对称[2 + 2]环成环制备环丁烯骨架
在此,我们介绍了一种钪催化合成策略,该策略提供了多种功能化的环丁烯骨架阵列,并以季碳中心装饰。这种方法扩大了2-炔基萘酚与烯烃的合成范围,为复杂分子结构的构建提供了一个通用的平台。不对称催化[2 + 2]环加成反应显示出广泛的底物范围和令人印象深刻的官能团耐受性,产率高达97%的产物效率高,对映体过量高达97%。扩大该过程的简单性,加上将这些环丁烯框架转化为各种取代产物的便利性,大大提高了该方法的合成效用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


