Synergistic Cu(II)/Amine-Catalyzed Cyclization of Enynone: Assembly of Tetralone and Tetrahydronaphthylimine
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An unprecedented synergistic copper- and amine-catalyzed cyclization of enynone is reported. This reaction features an efficient and straightforward construction of multisubstituted tetralone through an amine-assisted regioselective oxygen atom transfer process and stereoselective intramolecular Michael addition cyclization. Under dehydrative reaction conditions, the synthesis of tetrahydronaphthylimine derivatives with ketone group tolerance is achieved, which could be challenging via traditional methods. The utility of this reaction is demonstrated by further transformations of obtained molecules toward a variety of valuable multisubstituted naphthyl skeletons.
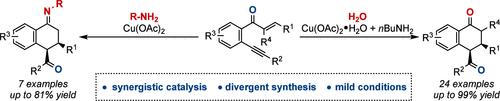
Cu(II)/胺协同催化的炔环化:四氢萘胺和四氢萘胺的组装
一个前所未有的协同铜和胺催化环化的炔报道。该反应的特点是通过胺辅助的区域选择性氧原子转移过程和立体选择性分子内迈克尔加成环化,有效而直接地构建了多取代四酮。在脱水反应条件下,合成了具有酮基耐受性的四氢萘亚胺衍生物,这是传统方法所无法实现的。该反应的效用被进一步转化为各种有价值的多取代萘基骨架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


