Cohesive, Vibrational, and Chemical Bonding Features of 3d- and 4d-Transition Metal MX (X = C, N) Compounds: A Systematic Approach
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
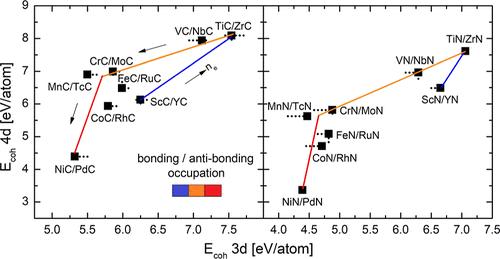
The paper presents an ab initio approach to the cohesive, vibrational, and phase stability properties of the MX (cF8) NaCl-type structure compounds formed by M = Sc to Ni of the 3d-transition-metal series and M = Y to Pd of the 4d-transition-metal series, with X = C and N. The cohesive energy (Ecoh) and a characteristic energy [E(0)] related to the vibrational entropy show a well-defined pattern of covariation with the average number of electrons per atom of the compound (ne). Various types of homologous behavior of compounds formed by 3d- and 4d-elements from the same group of the periodic table are detected. In particular, a striking covariation of the Ecoh [and E(0)] values for MX compounds formed by the pairs of elements Sc/Y to Ni/Pd with the increase in ne is established. These various systematic findings are discussed in terms of chemical bonding features of the compounds, which include the hybridization of the p-electron states of C (or N) with the d-states of the transition metals. Using systematic calculations of the crystal Hamiltonian populations, a comprehensive explanation is developed, which relies on the progressive occupation of bonding and antibonding electronic orbitals.
三维和4d过渡金属MX (X = C, N)化合物的内聚、振动和化学键特征:系统方法
介绍一个从头开始的粘性方法,振动,和相位稳定性能的MX (cF8) NaCl-type结构化合物由M = Sc的倪3 d-transition-metal系列和M = Y的Pd 4 d-transition-metal系列,X = C和n .凝聚力能源(Ecoh)和特征(E(0))相关的振动熵显示一个定义良好的模式共变平均每个原子的电子数的化合物(ne)。由元素周期表同一族的3d和4d元素形成的化合物的各种类型的同源行为被检测到。特别是,由Sc/Y到Ni/Pd元素对形成的MX化合物的Ecoh[和E(0)]值随着ne的增加而显著共变。根据化合物的化学键特征讨论了这些不同的系统发现,其中包括C(或N)的p电子态与过渡金属的d电子态的杂化。通过对晶体哈密顿居群的系统计算,提出了一个全面的解释,该解释依赖于成键和反键电子轨道的逐渐占据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


