Two-Dimensional Confinement Effects on the Dynamical Aspects of CO Oxidation over Pt(111)
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study investigates the effects of two-dimensional (2D) confinement on CO oxidation reaction over Pt(111) surfaces, emphasizing the structural, electronic, and dynamical aspects of CO2 formation and desorption. Using pristine and single-defect hexagonal boron nitride (h-BN) and graphitic carbon nitride (g-C3N4) as overlayers, we found that confinement by nitrogen-vacancy h-BN (NV) induces a significant reduction, greater than that of graphene (Gr), in the activation energy barrier for CO2 formation. The confinement effect on adsorption depends on the reference states chosen for adsorption energy calculations. Reference states, such as stable or original intersurface gaps, can yield either positive or negative confinement energy values, indicating adsorption weakening or strengthening, respectively. Importantly, adsorption weakening correlates better with barrier reduction. Post-transition-state dynamical simulations of CO2 desorption revealed pronounced vibrational oscillations. NV and Gr confinement led to complex vibrational features, indicating stronger interactions between CO2 and the 2D cover. This study highlights the profound impact of 2D confinement on catalytic processes, providing insights into how 2D materials modulate the electronic structure and vibrational dynamics of reactions. The findings could guide the design of efficient catalysts for industrial applications, particularly in heterogeneous catalysis.
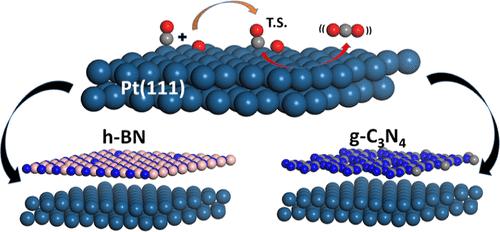
Pt(111)上CO氧化动力学方面的二维约束效应
本研究探讨了二维(2D)约束对铂(111)表面 CO 氧化反应的影响,强调了 CO2 生成和解吸的结构、电子和动力学方面。利用原始和单缺陷六方氮化硼(h-BN)和氮化石墨碳(g-C3N4)作为覆盖层,我们发现氮空位 h-BN (NV) 的约束作用显著降低了二氧化碳形成的活化能势垒,降低幅度大于石墨烯 (Gr)。对吸附的约束效应取决于吸附能计算所选择的参考状态。稳定或原始表面间隙等参考态可产生正或负的约束能值,分别表示吸附削弱或吸附增强。重要的是,吸附减弱与阻挡层减小有更好的相关性。二氧化碳解吸的过渡态后动力学模拟显示了明显的振动振荡。NV 和 Gr 限制导致了复杂的振动特征,表明 CO2 与二维覆盖层之间的相互作用更强。这项研究强调了二维约束对催化过程的深远影响,为二维材料如何调节反应的电子结构和振动动力学提供了见解。这些发现可以指导工业应用中高效催化剂的设计,特别是在异相催化方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


