Molecule-Based Proton–Electron Mixed Conductor with the Highest Ambipolar Conductivity
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
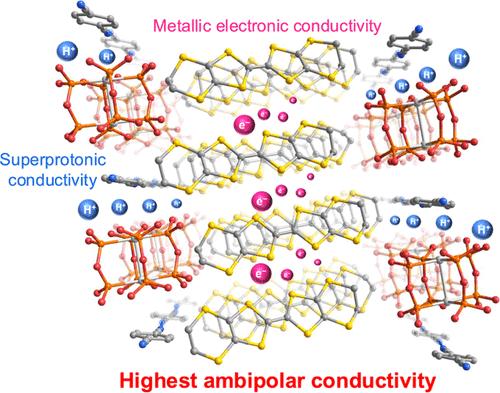
Proton–electron mixed conductors (PEMCs) are an essential component for potential applications in hydrogen separation and energy conversion devices. However, the exploration of PEMCs with excellent mixed conduction, which is quantified by the ambipolar conductivity, σamb = σeσH/(σe + σH) (σe: electronic conductivity; σH: proton conductivity), is still a great challenge, largely due to the lack of structural characterization of both conducting mechanisms. In this study, we prepared a molecule-based proton–electron mixed-conducting cation radical salt, (ET)4[Pt2(pop)2(Hpop)2]·PhCN (ET: bis(ethylenedithio)tetrathiafulvalene, pop2–: P2H2O52–), by electrocrystallization. The salt shows metallic electronic conduction, which arises from the (ET)2•+ layers, with a high σe value (1–2 S cm–1 at room temperature). The metallic state was corroborated by magnetic susceptibility measurement and band structure calculation. The salt also shows superprotonic conduction (σH = 2.1 × 10–2 S cm–1 at room temperature under dried conditions), which relies on the one-dimensional (1D) hydrogen-bonding network of protonated paddlewheel-type Pt-dimer complex anions, [Pt2(pop)2(Hpop)2]2–. Crystallographic and computational studies revealed the presence of infinite intra- and intermolecular O–H···O hydrogen bonds, which show a double-well-like potential energy curve with a negligible energy barrier in the 1D chain, facilitating Grotthuss-type proton hopping via Lewis basic sites. Among the structurally defined PEMCs, the present salt displays the highest room temperature σamb value of 2.1 × 10–2 S cm–1 even under dried conditions. The σamb value has the same order as those of reported perovskites-type metal oxides at high temperatures and achieves a level that is beneficial for the practical applications.
具有最高双极性电导率的分子基质子-电子混合导体
质子-电子混合导体(质子-电子混合导体)是氢分离和能量转换装置中潜在应用的重要部件。然而,对于具有优异混合导电性的pemc的探索,可以用双极性电导率来量化,σamb = σeσH/(σe + σH) (σe:电子导电性;σH:质子电导率),仍然是一个很大的挑战,很大程度上是由于缺乏两种导电机制的结构表征。在本研究中,我们通过电结晶法制备了一种基于分子的质子-电子混合导电阳离子基盐(ET)4[Pt2(pop)2(Hpop)2]·PhCN (ET:双(乙二硫代)四硫代烯,pop2 -: P2H2O52 -)。盐具有较高的σe值(室温下为1 ~ 2 S cm-1),表现出金属电子导电性。通过磁化率测量和能带结构计算证实了金属态。盐还表现出超质子传导(σH = 2.1 × 10-2 S cm-1,室温干燥条件下),这依赖于质子化浆轮型pt -二聚体配合阴离子[Pt2(pop)2(Hpop)2]2 -的一维(1D)氢键网络。晶体学和计算研究表明,分子内和分子间存在无限的O - h··O氢键,在一维链中具有可忽略的能量势垒,表现为双阱式势能曲线,促进了grotthuss型质子通过Lewis碱性位跳跃。在结构明确的PEMCs中,即使在干燥条件下,该盐的室温σamb值也最高,为2.1 × 10-2 S cm-1。在高温下,其σamb值与已有报道的钙钛矿型金属氧化物具有相同的数量级,达到了有利于实际应用的水平。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


