Computational exploration of the electrochemical oxidation mechanism of thiocyanate catalyzed by cobalt-phthalocyanines
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
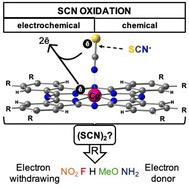
In this study, we focused on the mechanism of the electrocatalytic oxidation of thiocyanate, which in traditional electrodes typically requires high overpotentials. As models for reducing these overpotentials and catalyzing the reaction, we used a set of modified cobalt phthalocyanines (CoPc), known as electrocatalysts. Using DFT calculations, we explored how modifications to CoPc by adding electron-donating and withdrawing groups and the coordination of 4-amino thiophenol impact the oxidation process. The reaction mechanism for the electrooxidation of thiocyanate has remained elusive, where only the reaction products have been properly identified, including hydrogen cyanide and sulfate ions at pH 4. The approach for understanding the reaction was considering the formation of an (SCN)2 dimer as an intermediate that is a suitable precursor of the products of the reaction. Our findings showed that electron-donating groups and 4-amino thiophenol coordination lowered oxidation potentials, enhancing electrocatalytic efficiency and promoting thiocyanate radical formation and release before dimerization occurs. In contrast, electron-withdrawing groups facilitated dimerization while attached to cobalt, albeit with lower electrocatalytic proficiency. This study highlights the crucial role of CoPc modifications in thiocyanate oxidation, demonstrating the potential for improved electrocatalytic processes through tailored catalyst design.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


