Theoretical Insight into the Transition-Metal-Embedded Boron Nitride-Doped Graphene Single-Atom Catalysts for Electrochemical Nitrogen Reduction Reaction
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Single-atom catalysts (SACs) have become attractive options for the efficient nitrogen reduction reaction (NRR) because of their unique properties in the activation of nitrogen molecules. As a novel two-dimensional material, boron nitride (BN)-doped graphene has attracted much attention due to its electronic structure, which can be regulated with boron nitride coverage. In the current work, we first screened potential SACs for NRR from various single transition metal atoms embedded in BN-doped graphene (BNC) by using density functional theory (DFT) calculations. Excellent catalytic activity for NRR is demonstrated by the V, Mo, Ru, and Os anchored on the B vacancy and generated SACs, with overpotentials of −0.56, −0.52, −0.60, and −0.61 V vs the standard hydrogen electrode (SHE). Taking advantage of BN-doped graphene electronic structures that can be modified, we further investigated the effect of boron nitride coverage on the SACs’ NRR performance. The electronic structure of the metal center can be altered by controlling the boron nitride coverage, which can further affect the catalytic performance. The potential determining step (PDS) and also the maximal free energy difference vary by modulating the boron nitride coverage. A larger energy range than the hydrogen evolution reaction (HER) is covered by the maximum energy shift between the PDSs, which can reach 0.29 eV. This indicates that by changing the coverage of the BN of the substrate, it is expected to improve the SACs’s catalytic activity and selectivity of NRR. Moreover, it is possible for a pathway to change from one that is adsorption favorable to another one that is thermodynamically favorable of the intermediate NNH. Our results help to clarify the structure–performance correlations and expedite the creation of SACs for ammonia synthesis.
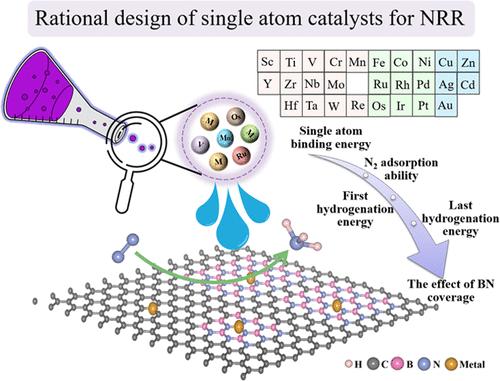
过渡金属嵌入氮化硼掺杂石墨烯单原子电化学氮还原反应催化剂的理论研究
单原子催化剂(SACs)由于其在氮分子活化方面的独特性能,已成为高效氮还原反应(NRR)中有吸引力的选择。氮化硼掺杂石墨烯作为一种新型的二维材料,由于其电子结构可以通过氮化硼的覆盖来调控而备受关注。在目前的工作中,我们首先通过密度泛函理论(DFT)计算,从嵌入bn掺杂石墨烯(BNC)中的各种单一过渡金属原子中筛选了NRR的潜在sac。V、Mo、Ru和Os锚定在B空位上并生成sac,与标准氢电极(SHE)相比,过电位分别为- 0.56、- 0.52、- 0.60和- 0.61 V,证明了NRR的优异催化活性。利用氮化硼掺杂石墨烯的可修饰电子结构,我们进一步研究了氮化硼覆盖对sac NRR性能的影响。通过控制氮化硼的覆盖可以改变金属中心的电子结构,进而影响催化性能。势决定步长(PDS)和最大自由能差随氮化硼覆盖率的变化而变化。pds之间的最大能量转移覆盖了比析氢反应(HER)更大的能量范围,可达0.29 eV。这表明通过改变底物BN的覆盖范围,有望提高SACs的催化活性和NRR的选择性。此外,一条途径有可能从一条有利于吸附的途径转变为另一条有利于中间NNH的热力学途径。我们的研究结果有助于澄清结构-性能的相关性,并加快氨合成SACs的创建。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


