Revealing the Potential-Dependent Rate-Determining Step of Oxygen Reduction Reaction on Single-Atom Catalysts
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Single-atom catalysts (SACs) have attracted widespread attention due to their potential to replace platinum-based catalysts in achieving efficient oxygen reduction reaction (ORR), yet the rational optimization of SACs remains challenging due to their elusive reaction mechanisms. Herein, by employing ab initio molecular dynamics simulations and a thermodynamic integration method, we have constructed the potential-dependent free energetics of ORR on a single iron atom catalyst dispersed on nitrogen-doped graphene (Fe–N4/C) and further integrated these parameters into a microkinetic model. We demonstrate that the rate-determining step (RDS) of the ORR on SACs is potential-dependent rather than invariant within the operative potential range. Specifically, under the charge-neutral condition, the RDS is calculated to be water desorption with the highest barrier, while as the potential increases, it gradually transitions to the protonation of *OH species, O2* species, and O* species, regardless of the protonation of *OH species as the potential-determining step. Moreover, we reveal the critical role of the dynamic adsorption of axially adsorbed water in facilitating the release of the single-atom site, thus enhancing the ORR rate. Our work has resolved the long-standing controversies over the RDS of ORR on SACs and implies that the step with the lowest exothermicity is not always synonymous with the RDS, highlighting the importance of examining the kinetic barriers under realistic potential conditions for understanding the electrocatalytic performance.
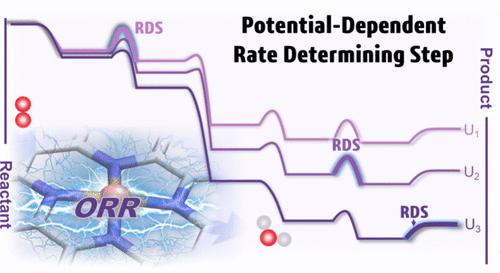
揭示单原子催化剂上氧还原反应电位依赖的速率决定步骤
单原子催化剂(SAC)因其可替代铂基催化剂实现高效氧还原反应(ORR)而受到广泛关注,但由于其反应机理难以捉摸,合理优化单原子催化剂仍具有挑战性。在此,我们通过采用原子分子动力学模拟和热力学整合方法,构建了分散在掺氮石墨烯(Fe-N4/C)上的单铁原子催化剂的氧还原反应随电势变化的自由能,并进一步将这些参数整合到微动力学模型中。我们证明,SAC 上 ORR 的速率决定步骤 (RDS) 与电势有关,而不是在工作电势范围内不变。具体来说,在电荷中性条件下,RDS 被计算为具有最高势垒的水解吸,而随着电势的增加,它逐渐过渡到 *OH 物种、O2* 物种和 O* 物种的质子化,而不管 *OH 物种的质子化是否为电势决定步骤。此外,我们还揭示了轴向吸附水的动态吸附在促进单原子位点释放从而提高 ORR 速率方面的关键作用。我们的研究工作解决了长期以来对 SAC 上 ORR 的 RDS 的争议,并暗示放热最低的步骤并不总是 RDS 的同义词,突出了在实际电位条件下研究动力学障碍对理解电催化性能的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


