Small CAG Repeat RNA Forms a Duplex Structure with Sticky Ends That Promote RNA Condensation
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Biomolecular condensation lays the foundation of forming biologically important membraneless organelles, but abnormal condensation processes are often associated with human diseases. Ribonucleic acid (RNA) plays a critical role in the formation of biomolecular condensates by mediating the phase transition through its interactions with proteins and other RNAs. However, the physicochemical principles governing RNA phase transitions, especially for short RNAs, remain inadequately understood. Here, we report that small CAG repeat (sCAG) RNAs composed of six to seven CAG repeats, which are pathogenic factors in Huntington’s disease, undergo phase transition in vitro and in cells. Leveraging solution nuclear magnetic resonance spectroscopy and advanced coarse-grained molecular dynamic simulations, we reveal that sCAG RNAs form duplex structures with 3′-sticky ends, where the GC stickers initiate intermolecular crosslinking and promote the formation of RNA condensates. Furthermore, we demonstrate that sCAG RNAs can form cellular condensates within nuclear speckles. Our work suggests that the RNA phase transition can be promoted by specific structural motifs, reducing the reliance on sequence length and multivalence. This opens avenues for exploring new functions of RNA in biomolecular condensates and designing novel biomaterials based on RNA condensation.
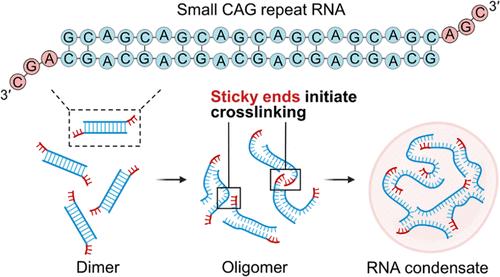
小CAG重复RNA形成具有粘性末端的双工结构,促进RNA缩聚
生物分子缩聚是形成生物学上重要的无膜细胞器的基础,但异常的缩聚过程往往与人类疾病有关。核糖核酸(RNA)通过与蛋白质和其他RNA的相互作用介导相变,在生物分子凝聚物的形成中起着至关重要的作用。然而,控制RNA相变的物理化学原理,特别是短RNA,仍然没有得到充分的理解。在这里,我们报道了由6到7个CAG重复序列组成的小CAG重复(sCAG) rna,它是亨廷顿病的致病因素,在体外和细胞中经历了相变。利用溶液核磁共振波谱和先进的粗粒度分子动力学模拟,我们发现sCAG RNA形成具有3 ' -粘性末端的双相结构,其中GC贴纸引发分子间交联并促进RNA凝聚物的形成。此外,我们证明了sCAG rna可以在核斑点内形成细胞凝聚物。我们的工作表明,RNA相变可以通过特定的结构基序来促进,从而减少对序列长度和多价性的依赖。这为探索RNA在生物分子凝聚体中的新功能和设计基于RNA凝聚体的新型生物材料开辟了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


