Rapid Assembly of 1,3-Dicarbonyl Fused 5-phenyl-1-Azabicyclo[3.1.0]hexanes and Their Cytotoxic Activities
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Synthesis of complex, multiring, spirocyclic, 1,3-dicarbonyl fused, and highly functionalized 5-phenyl-1-azabicyclo[3.1.0]hexanes (ABCH) has been achieved by an intermolecular reaction of 2-(2′-ketoalkyl)-1,3-indandiones or α,γ-diketo esters with (1-azidovinyl)benzenes under transition metal-free conditions. The reaction proceeds in a highly diastereoselective manner with a wide range of functional groups and provides moderate to good yields. Additionally, we have studied the preliminary biological activities of a few synthetic compounds against HeLa and triple-negative breast cancer (TNBC) cell lines.
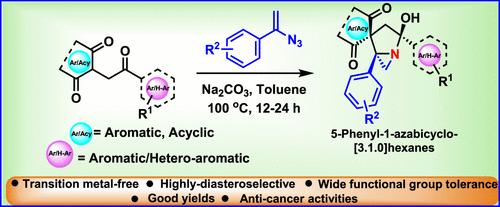
1,3-二羰基融合5-苯基-1-氮杂环[3.1.0]己烷的快速组装及其细胞毒活性
在无过渡金属条件下,通过 2-(2′-酮烷基)-1,3-茚二酮或 α,γ-二酮酯与 (1-azidovinyl)benzenes 的分子间反应,合成了复杂、多线、螺环、1,3-二羰基融合和高度官能化的 5-苯基-1-氮杂双环[3.1.0]己烷 (ABCH)。该反应以高度非对映选择性的方式进行,可涉及多种官能团,并提供中等至良好的产率。此外,我们还研究了一些合成化合物对 HeLa 和三阴性乳腺癌 (TNBC) 细胞系的初步生物活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


