"Suspended" Single Rhenium Atoms on Nickel Oxide for Efficient Electrochemical Oxidation of Glucose
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Well-defined single-atom catalysts (SACs) serve as ideal model systems for directly comparing experimental results with theoretical calculations, offering profound insights into heterogeneous catalytic processes. However, precisely designing and controllably synthesizing SACs remain challenging due to the unpredictable structure evolution of active sites and generation of embedded active sites, which may bring about steric hindrance during chemical reactions. Herein, we present the precious nonpyrolysis synthesis of Re SACs with a well-defined phenanthroline coordination supported by NiO (Re1-phen/NiO). Multiple experimental characterizations together with theoretical calculations unravel the idea that the isolated Re atoms are suspended on the NiO surface, connected by phenanthroline ligands standing perpendicular to the surface. This unique structure provides the Re1-phen/NiO SAC with a strong capability to activate glucose molecules, enabling fully exposed Re=O double bonds in an open-ended reaction environment to simultaneously react with hydroxyl and aldehyde groups at both ends of the glucose molecule, rapidly forming glucaric acid.
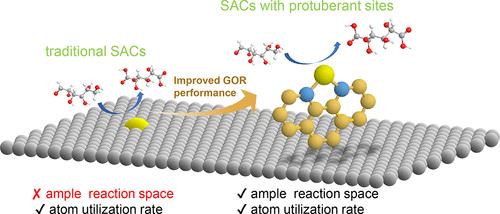
镍氧化物上“悬浮”单铼原子对葡萄糖的高效电化学氧化
定义良好的单原子催化剂(SACs)是直接比较实验结果和理论计算的理想模型系统,为多相催化过程提供了深刻的见解。然而,由于活性位点的结构演变不可预测,嵌入活性位点的产生可能会在化学反应中产生位阻,因此精确设计和可控合成SACs仍然具有挑战性。在此,我们提出了一种珍贵的非热解合成Re SACs的方法,该方法具有明确的NiO支持的菲罗啉配位(Re1-phen/NiO)。多个实验表征和理论计算揭示了孤立的Re原子悬浮在NiO表面上的想法,由垂直于表面的菲罗啉配体连接。这种独特的结构为Re1-phen/NiO SAC提供了强大的激活葡萄糖分子的能力,使得在开放式的反应环境中,完全暴露的Re=O双键可以同时与葡萄糖分子两端的羟基和醛基反应,快速形成葡萄糖酸。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


