Methanol-Enhanced Low-Cell-Voltage Hydrogen Generation at Industrial-Grade Current Density by Triadic Active Sites of Pt1–Pdn–(Ni,Co)(OH)x
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Methanol (ME) is a liquid hydrogen carrier, ideal for on-site-on-demand H2 generation, avoiding its costly and risky distribution issues, but this “ME-to-H2” electric conversion suffers from high voltage (energy consumption) and competitive oxygen evolution reaction. Herein, we demonstrate that a synergistic cofunctional Pt1Pdn/(Ni,Co)(OH)x catalyst with Pt single atoms (Pt1) and Pd nanoclusters (Pdn) anchored on OH-vacancy(VOH)-rich (Ni,Co)(OH)x nanoparticles create synergistic triadic active sites, allowing for methanol-enhanced low-voltage H2 generation. For MOR, OH* is preferentially adsorbed on Pdn and then interacts with the intermediates (such as *CHO or *CHOOH) adsorbed favorably on neighboring Pt1 with the assistance of hydrogen bonding from the surface hydrogen of (Ni,Co)(OH)x. The enhanced selectivity of the *CHOOH pathway, instead of *CO, sustains the MOR activity to a practically high current density. For HER, triadic Pt1, Pdn, and OH-vacancy sites on (Ni,Co)(OH)x create an “acid–base” microenvironment to facilitate water adsorption and splitting, forming H* species on Pt1 and Pdn, and *OH at the vacancy, to promote efficient H2 evolution from the asymmetric Pt1 and Pdn sites via the Tafel mechanism. The triadic-site synergy opens new avenues for the design and synthesis of highly efficient and stable cofunctional catalysts for “on-site-on-demand” H2 production, here facilitated by liquid methanol.
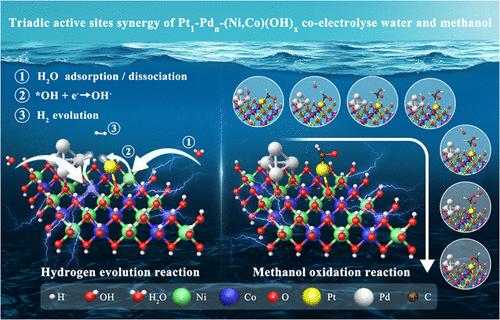
Pt1-Pdn - (Ni,Co)(OH)x三元活性位点在工业级电流密度下甲醇增强低压制氢
甲醇(ME)是一种液态氢载体,是按需现场制取 H2 的理想选择,可避免成本高昂且风险大的配送问题,但这种 "ME-H2 "电转化存在电压高(能耗大)和竞争性氧进化反应等问题。在此,我们证明了一种协同增效的 Pt1Pdn/(Ni,Co)(OH)x 共功能催化剂,其铂单原子(Pt1)和钯纳米团簇(Pdn)锚定在富含羟基空位(VOH)的(Ni,Co)(OH)x 纳米粒子上,形成了协同增效的三元活性位点,从而实现了甲醇增强型低压 H2 发电。在 MOR 中,OH* 优先吸附在 Pdn 上,然后在 (Ni,Co)(OH)x 表面氢键的帮助下,与邻近 Pt1 上吸附的中间产物(如 *CHO 或 *CHOOH)相互作用。*CHOOH途径而非*CO途径的选择性增强,使MOR活性持续到了实际上很高的电流密度。对于 HER 而言,(Ni,Co)(OH)x 上的三元 Pt1、Pdn 和 OH 空位创造了一个 "酸碱 "微环境,以促进水的吸附和分裂,在 Pt1 和 Pdn 上形成 H* 物种,在空位上形成 *OH,从而通过 Tafel 机制促进 H2 从不对称的 Pt1 和 Pdn 位点高效演化。三元位协同作用为设计和合成 "按需现场 "生产 H2 的高效、稳定的共功能催化剂开辟了新的途径,在这里,液态甲醇为催化剂提供了便利。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


