Organocatalytic Asymmetric Synthesis of o-Carboranyl Amines
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Carboranyl amines are distinct from typical organic amines. Due to the electronic influence of the carborane cage, they have low nucleophilicity and are reluctant to alkylate. Moreover, asymmetric synthesis of chiral carboranes is still in its infancy. Herein we have achieved the first catalytic asymmetric N-alkylation of o-carboranyl amine, providing general access to diverse secondary o-carboranyl amines with high efficiency and enantioselectivity under mild conditions. For the first time, asymmetric organocatalysis was introduced to carborane chemistry. Key to the success is the use of in situ generated (naphtho-)quinone methides as the alkylating reagents and suitable chiral acid catalysts. This protocol is also applicable to the asymmetric S-alkylation of 1-SH-o-C2B10H11. Control experiments and kinetic studies provided important insights into the reaction mechanism, which likely involves rate-determining generation of the quinone methide followed by fast and enantio-determining nucleophilic addition.
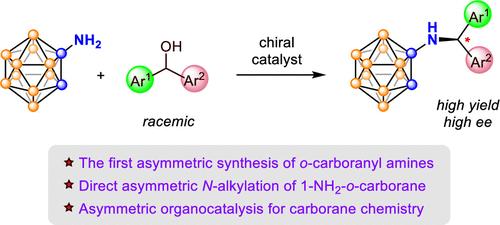
邻碳硼基胺的有机催化不对称合成
碳硼胺不同于典型的有机胺。由于碳硼烷笼的电子影响,它们亲核性低,不愿烷基化。此外,手性碳硼烷的不对称合成还处于起步阶段。本文首次实现了邻碳硼胺的催化不对称n -烷基化反应,为在温和条件下以高效率和对映选择性获得多种邻碳硼胺提供了途径。首次将不对称有机催化引入碳硼烷化学。成功的关键是使用了原位生成的萘醌作为烷基化试剂和合适的手性酸催化剂。该方法同样适用于1-SH-o-C2B10H11的不对称s烷基化反应。对照实验和动力学研究为反应机理提供了重要的见解,该反应可能涉及到快速和对映体确定的亲核加成的醌甲酰生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


