Evaluating the Dehydrogenation Performance of Cyclohexane on Pt-Skin AgPt3(111) and Ag3Pt(111) Surface Slabs: A Density Functional Theory Approach
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
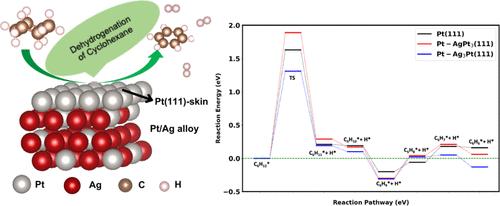
The development of highly effective dehydrogenation catalysts presents significant potential for storing hydrogen solutions with favorable economic advantages. In this study, we examined the dehydrogenation of cyclohexane on Pt-skin AgPt3(111) and Ag3Pt(111) surfaces in comparison with that on a Pt(111) pristine surface by applying density functional theory. We assessed the performance of various exchange–correlation functionals (PBE, BEEF-vdW, optPBE-vdW, and PBE-D3) in predicting the adsorption energy of cyclohexane on Pt–AgPt3(111), Pt–Ag3Pt(111), and Pt(111) surfaces and compared them to the experimental data. Through systematic calculations, we analyzed the electronic and structural properties of catalysts, adsorption energies of cyclohexane and intermediate molecules on various Ag–Pt alloy surfaces, surface charge distribution, dehydrogenation processes, and the effect of Ag concentration on its activity. The findings indicate that an increase in the Ag content leads to a closer shift of the d-band center of the Pt atom toward the Fermi level, moving from −2.31 to −1.81 eV. This shift increases surface charge accumulation. This gradual accumulation enhances the adsorption of cyclohexane. Notably, the dehydrogenation of cyclohexane on Pt-skin Ag3Pt exhibited a lower reaction energy, with a value of 1.31 eV compared to the pristine Pt(111) catalyst. This study revealed that the Pt-skin Ag3Pt(111) catalyst exhibits enhanced performance for the dehydrogenation of cyclohexane, which should stimulate additional experimental studies.
评价环己烷在pt -蒙皮AgPt3(111)和Ag3Pt(111)表面板上的脱氢性能:密度泛函理论方法
高效脱氢催化剂的开发具有巨大的储氢潜力和良好的经济效益。在本研究中,我们应用密度泛函理论研究了环己烷在Pt-skin AgPt3(111)和Ag3Pt(111)表面与Pt(111)原始表面上的脱氢。我们评估了各种交换相关函数(PBE、beefe - vdw、optbe - vdw和PBE- d3)预测环己烷在Pt - agpt3(111)、Pt - ag3pt(111)和Pt(111)表面的吸附能的性能,并将它们与实验数据进行了比较。通过系统计算,我们分析了催化剂的电子和结构性质、环己烷和中间分子在各种Ag - pt合金表面的吸附能、表面电荷分布、脱氢过程以及Ag浓度对其活性的影响。结果表明,Ag含量的增加导致Pt原子的d带中心向费米能级移动更近,从−2.31 eV移动到−1.81 eV。这种移动增加了表面电荷的积累。这种逐渐积累增强了对环己烷的吸附。值得注意的是,与原始Pt(111)催化剂相比,环己烷在Pt-skin Ag3Pt上脱氢的反应能较低,为1.31 eV。该研究表明,Pt-skin Ag3Pt(111)催化剂在环己烷脱氢反应中表现出更强的性能,值得进一步的实验研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


