A complementary experimental and computational study on methanol adsorption isotherms of H-ZSM-5
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
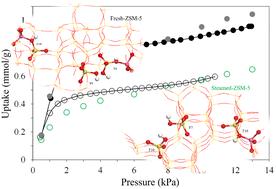
Methanol adsorption isotherms of fresh f-ZSM-5 and steamed s-ZSM-5 (Si/Al ≈ 40) are investigated experimentally at room temperature under equilibrium and by grand canonical Monte Carlo (GCMC) simulations with the aim of understanding the adsorption capacity, geometry and sites as a function of steam treatment (at 573 K for 24 h). Methanol adsorption energies calculated by GCMC are complemented by density functional theory (DFT) employing both periodic and quantum mechanics/molecular mechanics (QM/MM) techniques. Physical and textural properties of f-ZSM-5 and s-ZSM-5 are characterised by diffuse reflectance infrared Fourier transformed spectroscopy (DRIFTS) and N2-physisorption, which form a basis to construct models for f-ZSM-5 and s-ZSM-5 to simulate methanol adsorption isotherms by GCMC. Both Brønsted and silanol hydroxyls are observed in f-ZSM-5 and s-ZSM-5 by DRIFTS; however, these species, especially Brønsted species, decreased considerably upon steam treatment in s-ZSM-5 due to dealumination. Although the total pore volume and mesoporosity increased in s-ZSM-5 as compared in f-ZSM-5, the total surface area (375 m2 g−1) of the steamed zeolite is lower than the fresh zeolite (416 m2 g−1) due to pore plugging caused by partial dislodgement of framework Al on steam treatment. Implications of the steam treatment on the methanol adsorption capacity of the zeolites are reflected in the experimental methanol adsorption isotherms, collected (in the pressure range between 0 and 12 kPa) at room temperature under equilibrium, which find that the overall methanol uptake is lower for s-ZSM-5 than for f-ZSM-5. The GCMC simulations show that the nature, location and distribution of acidic hydroxyls determine the methanol adsorption capacity, geometry and hence the isotherm profiles of f-ZSM-5 and s-ZSM-5. The GCMC simulations provide insight into the different adsorption sites and their reactivity towards methanol which paves the way not only to describe the isotherms of f-ZSM-5 and s-ZSM-5 but also offers a means to understand better the deactivation of ZSM-5 by steam (leading to dealumination) and subtle differences in surface adsorbed species on ZSM-5 procured from different sources.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


