Associations Between Patient-Reported Nutritional Status, Toxicity, and Survival in Limited-Stage SCLC
IF 3.5
Q2 ONCOLOGY
引用次数: 0
Abstract
Introduction
In general, malnutrition is associated with more treatment toxicity and shorter survival in patients with cancer, but little is known about its impact on limited-stage (LS) SCLC. We investigated whether nutritional status and weight loss were associated with treatment outcomes in a randomized trial of thoracic radiotherapy (TRT) in LS SCLC (NCT02041845, N = 170).
Methods
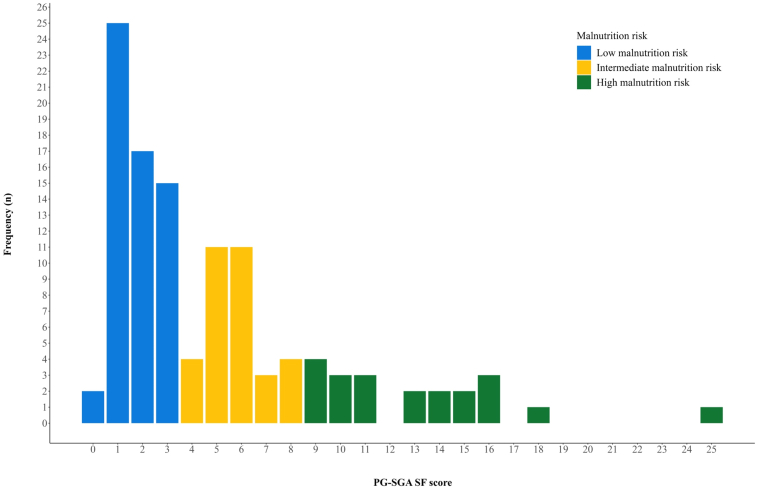
Patients received platinum-etoposide-chemotherapy and were randomized to receive TRT of 60 Gy in 40 fractions or 45 Gy in 30 fractions. They reported nutritional status on the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) and were categorized as having low (PG-SGA SF score 0–3), intermediate (score 4–8), or high (score ≥ 9) malnutrition risk.
Results
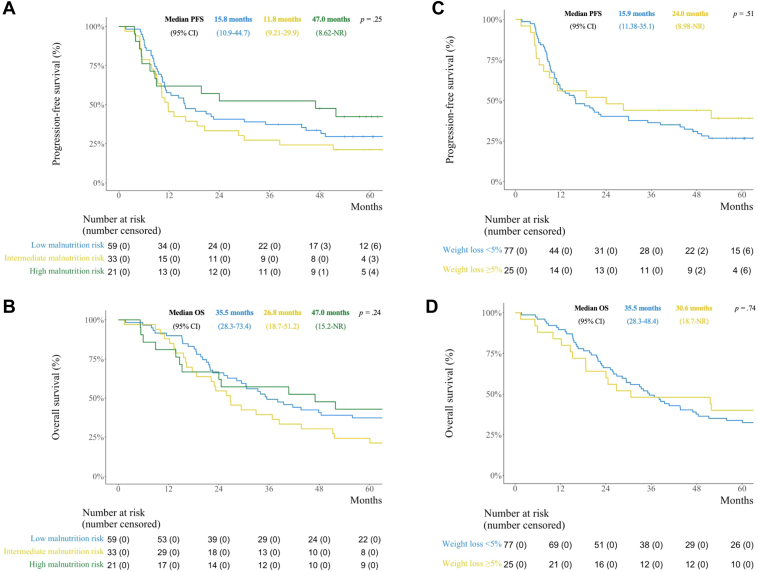
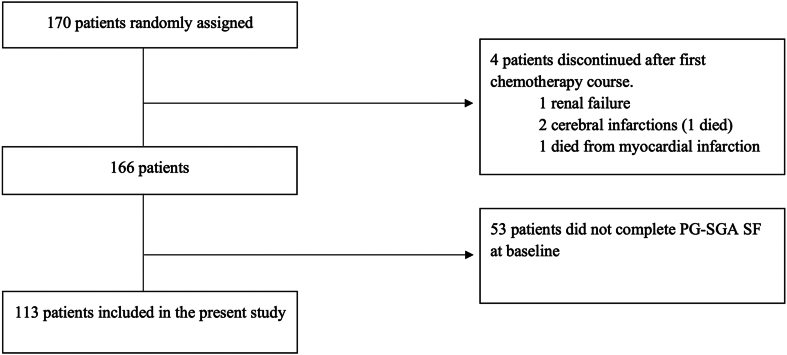
In total, 113 patients who completed the PG-SGA SF at baseline and received one or more fractions of TRT were analyzed. Median PG-SGA SF score was 3.0; 52.2% had low, 29.2% intermediate, and 18.6% had high malnutrition risk; and 22.1% had 5% or more weight loss three months before enrolment. There were no significant differences in grade 3 to 4 toxicity (low: 88.1%, intermediate: 90.9%, high: 85.7%; p = 0.86), median progression-free survival (low: 15.8 months, intermediate: 11.8 months, high: 47.0 months; p = 0.25) or median OS (low: 35.5 months, intermediate: 26.8 months, high: 47.0 months; p = 0.24) across malnutrition categories. Weight loss was not significantly associated with grade 3 to 4 toxicity (≥5%: 92.0%, <5%: 87.0%; p = 0.73), median progression-free survival (≥5%: 24.0 months, <5%: 15.9 months; p = 0.51) or median OS (≥5%: 30.6 months, <5%: 35.5 months; p = 0.74).
Conclusion
Patient-reported nutritional status and weight loss before concurrent chemoradiotherapy were neither associated with toxicity nor survival.
有限期SCLC患者报告的营养状况、毒性和生存之间的关系。
简介:一般来说,营养不良与癌症患者治疗毒性增加和生存期缩短有关,但营养不良对局限期(LS)SCLC 的影响却鲜为人知。在一项针对LS SCLC的胸部放疗(TRT)随机试验(NCT02041845,N = 170)中,我们研究了营养状况和体重下降是否与治疗效果相关:患者接受铂-依托泊苷化疗,并随机接受40次60 Gy或30次45 Gy的TRT治疗。他们用患者自制主观全面评估简表(PG-SGA SF)报告营养状况,并被分为低营养不良风险(PG-SGA SF 评分 0-3)、中营养不良风险(评分 4-8)或高营养不良风险(评分≥9):共对 113 名在基线完成 PG-SGA SF 并接受过一次或多次 TRT 治疗的患者进行了分析。PG-SGA SF评分中位数为3.0;52.2%为低营养不良风险,29.2%为中营养不良风险,18.6%为高营养不良风险;22.1%的患者在入组前三个月体重下降5%或以上。不同营养不良类别的 3 至 4 级毒性(低:88.1%,中:90.9%,高:85.7%;p = 0.86)、中位无进展生存期(低:15.8 个月,中:11.8 个月,高:47.0 个月;p = 0.25)或中位 OS(低:35.5 个月,中:26.8 个月,高:47.0 个月;p = 0.24)无明显差异。体重减轻与3至4级毒性(≥5%:92.0%,p = 0.73)、中位无进展生存期(≥5%:24.0个月,p = 0.51)或中位OS(≥5%:30.6个月,p = 0.74)无明显相关性:结论:患者报告的营养状况和同期放化疗前的体重减轻与毒性和生存期无关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


