Six pairs of α-pyrone meroterpenoid dimers from Hypericum monogynum with anti-neuroinflammatory activity†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-01-08
DOI:10.1039/d4qo02179g
引用次数: 0
Abstract
Hypericumonates A–F (), six pairs of new α-pyrone meroterpenoid dimers, along with four possible precursors () were isolated from the leaves and twigs of Hypericum monogynum. Hypericumonates A–C, the first α-pyrone meroterpenoid dimers with two motifs (a 6/6/4-6/6 ring system) connected via C-8–C-9, were proposed via [2 + 2] cycloaddition of α-pyrone meroterpenoid. Their structures were determined by spectroscopic analysis, quantum chemical calculations, and single-crystal X-ray diffraction analysis. From biosynthesis analysis, five pairs of new α-pyrone meroterpenoid dimers () are derived from a new isopentenyl-α-pyrone (). Compounds is an unusual homodimer, whereas compounds and represent rare heterodimers. Inspired by the traditional anti-inflammatory usages of H. monogynum, all dimers except for were discovered to show good NO inhibitory effect with IC50 values of 2.41 ± 0.31 μM to 14.25 ± 1.93 μM, better than the positive control, minocycline (IC50: 19.09 ± 1.34 μM). Further mechanistic study implied that (+)- could prohibit the expression of iNOS and COX-2 in BV-2 cells, and the molecular docking study implied the possible interaction between (+)-/(−)- and these two proteins.

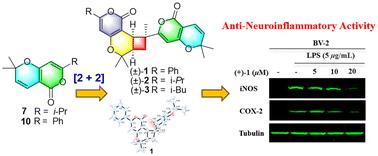
具有抗神经炎活性的金丝桃六对α-吡酮类二聚体
从金丝桃(Hypericum monogynum)的叶片和枝条中分离到6对新的α-吡酮二萜类二聚体A-F(1-6)和4个可能的前体(7-10)。采用[2 + 2]环加成法制备了首个具有6/6/4-6/6环结构的α-吡酮-梅罗萜类二聚体a - c。通过光谱分析、量子化学计算和单晶x射线衍射分析确定了它们的结构。从生物合成分析中,从一个新的异戊烯基α-吡咯酮(7)中得到5对新的α-吡咯酮二萜类二聚体(1 - 5)。化合物2为罕见的同二聚体,而化合物1和3-6为罕见的异二聚体。受传统抗炎作用的启发,除6外,其余二聚体均表现出良好的NO抑制作用,IC50值为2.41±0.31 μM ~ 14.25±1.93 μM,优于阳性对照米诺环素(IC50值为19.09±1.34 μM)。进一步的机制研究表明(+)-1可以抑制BV-2细胞中iNOS和COX-2的表达,分子对接研究表明(+)-1/(−)-1可能与这两种蛋白相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


