Stereodivergent Synthesis of the Vicinal Difluorinated Tetralin of Casdatifan Enabled by Ru-Catalyzed Transfer Hydrogenation
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We disclose a stereodivergent strategy to prepare vicinal difluorinated tetralins from γ-substituted tetralones via a combination of catalyst-controlled transfer hydrogenation and substrate-controlled fluorinations. This process is easily scalable and amenable to highly functionalized substrates, as demonstrated here in the late-stage synthesis of casdatifan, a clinical-stage inhibitor of hypoxia-inducible factor-2α. Analysis of the physicochemical properties of casdatifan, which features a cis-vicinal difluoride, revealed a higher level of facial polarization compared to its trans-vicinal difluoride isomers.
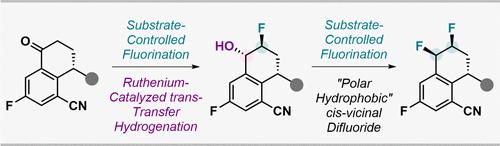
通过 Ru 催化的转移加氢反应立体异构合成 Casdatifan 的双侧二氟化四氢萘
我们揭示了一种立体发散策略,通过催化剂控制的转移氢化和底物控制的氟化相结合,从γ-取代的四氟酮制备邻近的二氟化四氟素。这个过程很容易扩展,并且适用于高度功能化的底物,正如这里在缺氧诱导因子2α的临床阶段抑制剂casdatifan的后期合成中所证明的那样。卡达地凡的理化性质分析表明,其具有顺邻二氟化特征,与其跨邻二氟化异构体相比,其面部极化水平更高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


