Centromere inactivation during aging can be rescued in human cells
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Aging involves a range of genetic, epigenetic, and physiological alterations. A key characteristic of aged cells is the loss of global heterochromatin, accompanied by a reduction in canonical histone levels. In this study, we track the fate of centromeres in aged human fibroblasts and tissues and in various cellular senescent models. Our findings reveal that the centromeric histone H3 variant CENP-A is downregulated in aged cells in a p53-dependent manner. We observe repression of centromeric noncoding transcription through an epigenetic mechanism via recruitment of a lysine-specific demethylase 1 (LSD1/KDM1A) to centromeres. This suppression results in defective de novo CENP-A loading at aging centromeres. By dual inhibition of p53 and LSD1/KDM1A in aged cells, we mitigate the reduction in centromeric proteins and centromeric transcripts, leading to the mitotic rejuvenation of these cells. These results offer insights into a unique mechanism for centromeric inactivation during aging and provide potential strategies to reactivate centromeres.
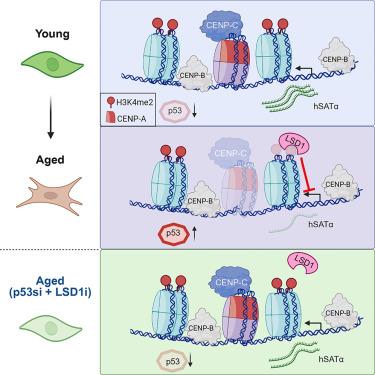
人类细胞在衰老过程中可以挽救着丝粒失活
衰老包括一系列遗传、表观遗传和生理上的改变。衰老细胞的一个关键特征是整体异染色质的丧失,伴随着标准组蛋白水平的降低。在这项研究中,我们在衰老的人类成纤维细胞和组织以及各种细胞衰老模型中追踪着丝粒的命运。我们的研究结果表明,着丝粒组蛋白H3变体CENP-A在衰老细胞中以p53依赖的方式下调。我们观察到着丝粒非编码转录的抑制是通过表观遗传机制通过赖氨酸特异性去甲基酶1 (LSD1/KDM1A)募集到着丝粒。这种抑制导致老化着丝粒中有缺陷的新生CENP-A负载。通过在衰老细胞中双重抑制p53和LSD1/KDM1A,我们减轻了着丝粒蛋白和着丝粒转录物的减少,导致这些细胞的有丝分裂再生。这些结果提供了对衰老过程中着丝粒失活的独特机制的见解,并提供了重新激活着丝粒的潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


