Photocatalytic selective oxidation of glycerol to formic acid and formaldehyde over surface cobalt-doped titanium dioxide
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Glycerol is one of the most important biomass platform compounds that is a by-product of biodiesel production, and the selective cleavage of the C![]() C bond of glycerol to produce liquid hydrogen carriers (i.e., formic acid and formaldehyde) offers a viable strategy to alleviate the currently faced energy shortages. However, the harsh reaction conditions, long reaction times, poor yields of liquid hydrogen carriers, and the tendency of peroxidation to carbon dioxide (CO2) make the selective C
C bond of glycerol to produce liquid hydrogen carriers (i.e., formic acid and formaldehyde) offers a viable strategy to alleviate the currently faced energy shortages. However, the harsh reaction conditions, long reaction times, poor yields of liquid hydrogen carriers, and the tendency of peroxidation to carbon dioxide (CO2) make the selective C![]() C bond cleavage of glycerol more challenging. Herein, we report the selective C
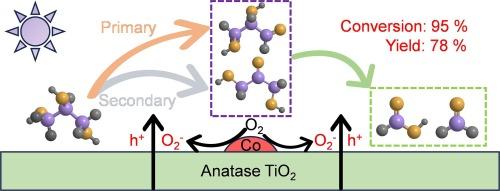
C bond cleavage of glycerol more challenging. Herein, we report the selective C![]() C bond cleavage of glycerol to formic acid and formaldehyde using surface cobalt (Co)-doped titanium dioxide (TiO2) under light irradiation and ambient conditions. The optimized system exhibits a high conversion of glycerol (95 %) and the yield of liquid hydrogen carriers reaches 78 % (formic acid, 57 %; formaldehyde, 21 %) in 8 h, effectively preventing their peroxidation to CO2. The outstanding photocatalytic performance is mainly attributed to the introduction of Co species and oxygen vacancies that promote the separation of photogenerated charges and holes and provide more adsorption sites for oxygen which are electron acceptors, respectively. In-depth investigations have shown that photogenerated holes and superoxide radicals are the main active species in this reaction. This work presents an effective and promising strategy for the efficient utilization of biomass resources.
C bond cleavage of glycerol to formic acid and formaldehyde using surface cobalt (Co)-doped titanium dioxide (TiO2) under light irradiation and ambient conditions. The optimized system exhibits a high conversion of glycerol (95 %) and the yield of liquid hydrogen carriers reaches 78 % (formic acid, 57 %; formaldehyde, 21 %) in 8 h, effectively preventing their peroxidation to CO2. The outstanding photocatalytic performance is mainly attributed to the introduction of Co species and oxygen vacancies that promote the separation of photogenerated charges and holes and provide more adsorption sites for oxygen which are electron acceptors, respectively. In-depth investigations have shown that photogenerated holes and superoxide radicals are the main active species in this reaction. This work presents an effective and promising strategy for the efficient utilization of biomass resources.
光催化选择性氧化甘油为甲酸和甲醛表面钴掺杂二氧化钛。
甘油是生物柴油生产过程中最重要的生物质平台化合物之一,通过选择性裂解甘油的CC键来生产液氢载体(即甲酸和甲醛)为缓解当前面临的能源短缺提供了一种可行的策略。然而,恶劣的反应条件、较长的反应时间、较差的液氢载体产率以及过氧化为二氧化碳(CO2)的倾向使得甘油的选择性CC键裂解更具挑战性。本文报道了在光照和环境条件下,使用表面掺杂钴(Co)的二氧化钛(TiO2)选择性地将甘油裂解成甲酸和甲醛的CC键。优化后的体系甘油转化率高(95%),液氢载体收率达78%(甲酸为57%;甲醛含量21%),有效防止其过氧化为CO2。优异的光催化性能主要归功于Co和氧空位的引入,它们促进了光生电荷和空穴的分离,并为作为电子受体的氧提供了更多的吸附位点。深入研究表明,光生空穴和超氧自由基是该反应的主要活性物质。本研究为生物质资源的高效利用提供了一种有效的、有前景的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies
文献相关原料
公司名称
产品信息
麦克林
1,4-benzoquinone
麦克林
isopropanol
麦克林
formic acid
麦克林
glyceraldehyde
麦克林
dihydroxyacetone
阿拉丁
2,4-dinitrophenylhydrazine
阿拉丁
melamine
阿拉丁
triethanolamine
阿拉丁
formaldehyde
阿拉丁
glycolic acid
阿拉丁
TiO<sub>2</sub> (Rutile)
阿拉丁
TiO<sub>2</sub> (Anatase)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


