Mesoporous Co-MoS2 with sulfur vacancies: A bifunctional electrocatalyst for enhanced water-splitting reactions in alkaline media
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
As a graphene-like layered material, molybdenum disulfide (MoS2), has attracted increasing attentions for its promising application in electrocatalysis. Whereas MoS2 still suffers from the sluggish reaction kinetics in oxygen evolution reaction (OER) due to the low density of active sites in most exposed planes. In this work, high density of active sites on MoS2 basal planes has been obtained by synthesizing mesoporous MoS2 with Co doping and sulfur vacancies (VS). The synergy of the mesoporous structure, Co doping, and sulfur vacancies resulted in optimized bifunctional electrocatalytic activity for both hydrogen evolution reaction (HER) and OER in alkaline media. The overpotential required to achieve a current density of 10 mA cm−2 (denoted as η10) is 34 mV for HER and 268 mV for OER, respectively. The two-electrode electrolyzer constructed with the as-prepared cobalt-doped mesoporous MoS2 electrodes exhibited a low bias (η10 = 1.58 V) for overall water splitting. Density functional theory (DFT) calculations confirm the significance of Co doping and the S vacancy defects, which lowers the Gibbs free energy (ΔG) for the formation of the corresponding intermediates.
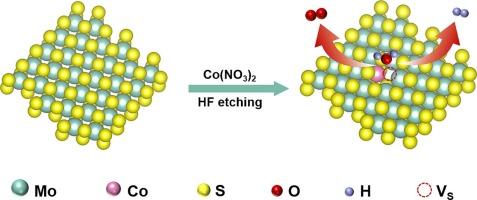
具有硫空位的介孔Co-MoS2:在碱性介质中增强水分解反应的双功能电催化剂。
二硫化钼(MoS2)作为一种类石墨烯的层状材料,因其在电催化方面的应用前景而受到越来越多的关注。而二硫化钼在出氧反应(OER)中,由于大部分暴露平面的活性位点密度低,反应动力学仍然缓慢。在本研究中,通过Co掺杂和硫空位(VS)合成介孔MoS2,在MoS2基面上获得了高密度的活性位点。介孔结构、Co掺杂和硫空位的协同作用优化了碱性介质中析氢反应(HER)和OER的双功能电催化活性。达到10 mA cm-2电流密度所需的过电位(记为η10), HER为34 mV, OER为268 mV。用钴掺杂介孔二硫化钼电极构建的双电极电解槽具有低偏置(η10 = 1.58 V)的整体水分解性能。密度泛函理论(DFT)计算证实了Co掺杂和S空位缺陷的重要性,这降低了相应中间体形成的吉布斯自由能(ΔG)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


