Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Triflates with Alkyl Halides: Mechanism-Informed Design of More General Conditions
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
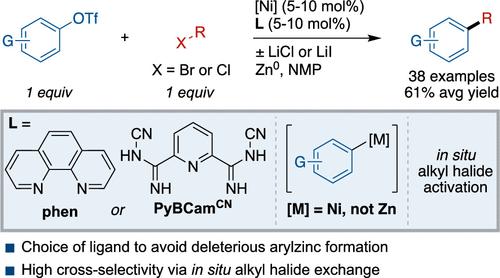
Aryl triflates make up a class of aryl electrophiles that are available in a single step from the corresponding phenol. Despite the known reactivity of nickel complexes for aryl C–O bond activation of phenol derivatives, nickel-catalyzed cross-electrophile coupling using aryl triflates has proven challenging. Herein, we report a method to form C(sp2)–C(sp3) bonds by coupling aryl triflates with alkyl bromides and chlorides using phenanthroline (phen) or pyridine-2,6-bis(N-cyanocarboxamidine) (PyBCamCN)-ligated nickel catalysts. The scope of the reaction is demonstrated with 38 examples (61 ± 14% average yield). Mechanistic studies provide a rationale for the conditions used and a roadmap for further applications of cross-electrophile coupling. First, the rate of alkyl radical generation is controlled by maintaining the majority of alkyl halide as the alkyl chloride, which is unreactive, and utilizing a dynamic halide exchange process to adjust the concentration of reactive alkyl bromide or iodide. Second, the challenge of using electron-rich aryl triflates appears to be due to off-cycle transmetalation to form unproductive aryl zinc reagents. The optimal PyBCamCN ligand together with LiCl avoids this deleterious transmetalation step.
镍催化的芳基三氟酸酯与卤代烷的交叉亲电偶联:更一般条件下的机理设计
芳基三氟酸酯构成一类芳基亲电试剂,从相应的苯酚一步即可得到。尽管已知镍配合物对苯酚衍生物的芳基C-O键活化的反应性,但镍催化的芳基三氟酸酯的交叉亲电偶联已被证明具有挑战性。本文报道了一种以邻菲罗啉(phen)或吡啶-2,6-双(n -氰基carboxamidine) (PyBCamCN)为连接镍催化剂,将三氟化芳基酸酯与烷基溴化物和氯化物偶联形成C(sp2) -C (sp3)键的方法。38个样品(平均产率61±14%)证明了该反应的范围。机理研究为所使用的条件提供了基本原理,并为进一步应用交叉亲电偶联提供了路线图。首先,控制烷基自由基的生成速率,方法是保持大部分的烷基卤化物为不活泼的烷基氯,并利用动态卤化物交换过程来调节活泼的烷基溴或碘化物的浓度。其次,使用富电子芳基三氟酸盐的挑战似乎是由于非循环转化形成非生产性芳基锌试剂。最佳的PyBCamCN配体与LiCl一起避免了这一有害的转化步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


