Catalyst-Free Nitrogen Fixation by Microdroplets through a Radical-Mediated Disproportionation Mechanism under Ambient Conditions
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nitrogen fixation is essential for the sustainable development of both human society and the environment. Due to the chemical inertness of the N≡N bond, the traditional Haber–Bosch process operates under extreme conditions, making nitrogen fixation under ambient conditions highly desirable but challenging. In this study, we present an ultrasonic atomizing microdroplet method that achieves nitrogen fixation using water and air under ambient conditions in a rationally designed sealed device, without the need for any catalyst. The total nitrogen fixation rate achieved is 6.99 μmol/h, yielding ammonium as the reduction product and nitrite and nitrate as the oxidation products, with hydrogen peroxide produced as a byproduct at a rate of 4.29 μmol/h. Using electron paramagnetic resonance (EPR) spectroscopy, we captured reactive species, including hydrogen, hydroxyl, singlet oxygen, superoxide anion, and NO radicals. In conjunction with in situ mass spectrometry (MS) and isotope labeling, we confirmed the presence of nitrogen-containing intermediates, such as HN═NOH+•, H2N–N(OH)2+•, HNO+, and NH2OH+•. Supported by these findings and theoretical calculations, we propose a radical-mediated nitrogen disproportionation mechanism. Simulations of naturally occurring condensed microdroplets also demonstrated nitrogen redox fixation. This microdroplet-based method not only offers a potential pathway for nitrogen fixation in practical applications and sustainable development but also deepens our understanding of the natural nitrogen cycle.
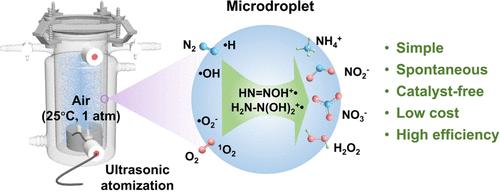
环境条件下微滴通过自由基介导的歧化机制进行无催化剂固氮
固氮对人类社会和环境的可持续发展至关重要。由于N≡N键的化学惰性,传统的Haber-Bosch工艺在极端条件下运行,使得在环境条件下的固氮非常理想,但具有挑战性。在本研究中,我们提出了一种超声波雾化微滴法,在合理设计的密封装置中,在环境条件下利用水和空气实现固氮,不需要任何催化剂。总固氮速率为6.99 μmol/h,还原产物为铵,氧化产物为亚硝酸盐和硝酸盐,副产物为过氧化氢,速率为4.29 μmol/h。利用电子顺磁共振(EPR)光谱,我们捕获了活性物质,包括氢、羟基、单线态氧、超氧阴离子和NO自由基。结合原位质谱(MS)和同位素标记,我们证实了含氮中间体的存在,如HN = NOH+•、H2N-N (OH)2+•、HNO+和NH2OH+•。在这些发现和理论计算的支持下,我们提出了自由基介导的氮歧化机制。模拟自然发生的冷凝微滴也证明了氮氧化还原固定。这种基于微滴的方法不仅为固氮的实际应用和可持续发展提供了一条潜在的途径,而且加深了我们对自然氮循环的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


