Copper Chelate Targeting Externalized Phosphatidylserine Inhibits PD-L1 Expression and Enhances Cancer Immunotherapy
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Inhibitors of the PD-1/PD-L1 immune checkpoint have revolutionized cancer treatment. However, the clinical response remains limited, with only 20% of patients benefiting from treatment and approximately 60% of PD-L1-positive patients exhibiting resistance. One key factor contributing to resistance is the externalization of phosphatidylserine (PS) on the surface of cancer cells, which suppresses immune responses and promotes PD-L1 expression, further hindering the efficacy of PD-L1 blockade therapies. Here, we introduce a copper chelate composed of a terpyridine–Cu complex with a farnesol tail designed to selectively target and cap the externalized PS on cancer cells. This approach not only promotes dendritic cell maturation and effector T-cell proliferation and tumor infiltration but also significantly inhibits PD-L1 expression, thereby amplifying T-cell-mediated immune responses. Our results demonstrate that this strategy induces robust immunological memory and leads to the eradication of tumors in over 70% of mice with colorectal and melanoma cancers. These findings highlight a promising, antibody-independent strategy for cancer immunotherapy where targeting externalized PS could overcome current limitations of checkpoint blockade therapies.
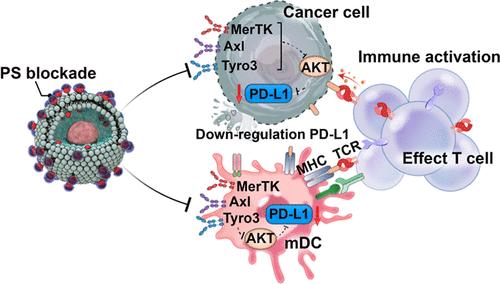
靶向外源性磷脂酰丝氨酸的铜螯合物抑制PD-L1表达并增强癌症免疫治疗
PD-1/PD-L1免疫检查点抑制剂已经彻底改变了癌症治疗。然而,临床反应仍然有限,只有20%的患者从治疗中受益,大约60%的pd - l1阳性患者表现出耐药性。导致耐药的一个关键因素是磷脂酰丝氨酸(PS)在癌细胞表面的外化,它抑制免疫反应并促进PD-L1的表达,进一步阻碍了PD-L1阻断疗法的疗效。在这里,我们介绍了一种铜螯合物,它由三吡啶- cu络合物和法尼醇尾部组成,旨在选择性地靶向和封盖癌细胞上的外化PS。该方法不仅促进树突状细胞成熟、效应t细胞增殖和肿瘤浸润,而且显著抑制PD-L1的表达,从而放大t细胞介导的免疫应答。我们的研究结果表明,这种策略诱导了强大的免疫记忆,并导致超过70%的结直肠癌和黑色素瘤小鼠肿瘤根除。这些发现强调了一种有希望的、不依赖抗体的癌症免疫治疗策略,其中靶向外化PS可以克服当前检查点阻断疗法的局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


