Cooperative Anion−π Catalysis with Chiral Molecular Cages toward Enantioselective Desymmetrization of Anhydrides
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Exploiting novel noncovalent interactions for catalysis design represents a fascinating direction. For the flexible and relatively weak anion−π interactions, manipulation of two or more π-acidic surfaces for cooperative activation is highly desirable. Here, we demonstrate the strategy of cooperative anion−π catalysis based on chiral molecular cages with V-shaped electron-deficient cavities for synergic binding and activation of dicarbonyl electrophiles toward highly enantioselective desymmetrization transformation. The chiral cages were readily synthesized by incorporation of additional chiral base sites in one step. The cages efficiently catalyzed methanolytic desymmetrization of a series of meso cyclic anhydrides in nearly quantitative yields and up to 94% ee. In contrast, the non-cage analogues and simple control catalysts showed sluggish conversion and much lower enantioselectivity. Crystal structure, substrate binding studies, and theoretical modeling consistently suggested the essential role of the cage electron-deficient cavities in harnessing cooperative anion−π interactions for efficient activation and excellent selectivity control.
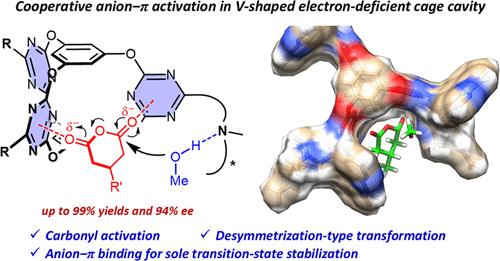
手性分子笼阴离子- π协同催化酸酐对映选择性去对称
开发新的非共价相互作用的催化设计代表了一个迷人的方向。对于灵活和相对弱的阴离子- π相互作用,操纵两个或多个π-酸性表面进行协同活化是非常可取的。在这里,我们展示了基于带有v型缺电子腔的手性分子笼的阴离子−π协同催化策略,用于协同结合和激活二羰基亲电试剂,以实现高度对映选择性的去对称转化。手性笼很容易通过加入额外的手性碱基位点一步合成。该笼有效地催化了一系列中环酸酐的甲醇解不对称反应,收率接近定量,ee高达94%。相比之下,非笼型类似物和简单对照催化剂的转化缓慢,对映体选择性低得多。晶体结构、底物结合研究和理论建模一致表明,笼型缺电子腔在利用阴离子−π相互作用实现高效活化和卓越的选择性控制方面发挥了重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


